| 1 |
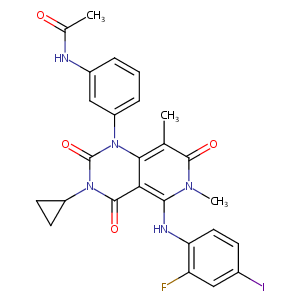
ClinicalTrials.gov (NCT03704688) Trial of Trametinib and Ponatinib in Patients With KRAS Mutant Advanced Non-Small Cell Lung Cancer
|
| 2 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6495).
|
| 3 |
Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015
|
| 4 |
Coronaviruses - drug discovery and therapeutic options. Nat Rev Drug Discov. 2016 May;15(5):327-47.
|
| 5 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 5890).
|
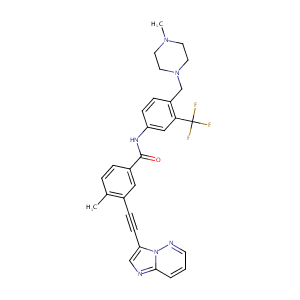
| 6 |
Ponatinib FDA Label
|
| 7 |
Radium 223 dichloride for prostate cancer treatment. Drug Des Devel Ther. 2017 Sep 6;11:2643-2651.
|
| 8 |
Combination small molecule MEK and PI3K inhibition enhances uveal melanoma cell death in a mutant GNAQ- and GNA11-dependent manner. Clin Cancer Res. 2012 Aug 15;18(16):4345-55. doi: 10.1158/1078-0432.CCR-11-3227. Epub 2012 Jun 25.
|
| 9 |
Harnessing autophagy to overcome mitogen-activated protein kinase kinase inhibitor-induced resistance in metastatic melanoma. Br J Dermatol. 2019 Feb;180(2):346-356. doi: 10.1111/bjd.17333. Epub 2018 Nov 25.
|
| 10 |
Physapubescin B enhances the sensitivity of gastric cancer cells to trametinib by inhibiting the STAT3 signaling pathway. Toxicol Appl Pharmacol. 2020 Dec 1;408:115273. doi: 10.1016/j.taap.2020.115273. Epub 2020 Oct 6.
|
| 11 |
Clinical resistance associated with a novel MAP2K1 mutation in a patient with Langerhans cell histiocytosis. Pediatr Blood Cancer. 2018 Sep;65(9):e27237. doi: 10.1002/pbc.27237. Epub 2018 May 16.
|
| 12 |
DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2018 Jan 4;46(D1):D1074-D1082. (ID: DB08901)
|
| 13 |
Evaluation of the effect of multiple doses of rifampin on the pharmacokinetics and safety of ponatinib in healthy subjects. Clin Pharmacol Drug Dev. 2015 Sep;4(5):354-60.
|
| 14 |
Novel pathways of ponatinib disposition catalyzed by CYP1A1 involving generation of potentially toxic metabolites. J Pharmacol Exp Ther. 2017 Oct;363(1):12-19.
|
| 15 |
Potential of ponatinib to treat chronic myeloid leukemia and acute lymphoblastic leukemia. Onco Targets Ther. 2013 Aug 20;6:1111-8.
|
|
|
|
|
|
|