| 1 |
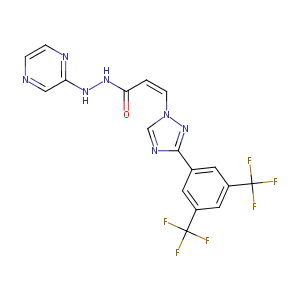
ClinicalTrials.gov (NCT05952687) Trial of Idasanutlin and Selinexor Therapy for Children With Progressive/Relapsed AT/RT or Extra-CNS Malignant Rhabdoid Tumors
|
| 2 |
Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015
|
| 3 |
Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA)
|
| 4 |
Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA)
|
| 5 |
ClinicalTrials.gov (NCT04349098) Evaluation of Activity and Safety of Oral Selinexor in Participants With Severe COVID-19 Infection. U.S. National Institutes of Health.
|
| 6 |
ClinicalTrials.gov (NCT02025985) Phase II Study of KPT-330 (Selinexor) in Female Patients With Advanced Gynaecologic Malignancies. U.S. National Institutes of Health.
|
| 7 |
ClinicalTrials.gov (NCT02545283) A Study of Idasanutlin With Cytarabine Versus Cytarabine Plus Placebo in Participants With Relapsed or Refractory Acute Myeloid Leukemia (AML) (MIRROS). U.S. National Institutes of Health.
|
| 8 |
Small molecules, big targets: drug discovery faces the protein-protein interaction challenge.Nat Rev Drug Discov. 2016 Aug;15(8):533-50.
|
| 9 |
ClinicalTrials.gov (NCT02407080) Open Label Study of Single Agent Oral RG7388 in Patients With Polycythemia Vera and Essential Thrombocythemia. U.S. National Institutes of Health.
|
| 10 |
Inhibition of CRM1-dependent nuclear export sensitizes malignant cells to cytotoxic and targeted agents. Semin Cancer Biol. 2014 August; 0: 62-73.
|
| 11 |
FDA label of Selinexor. The 2020 official website of the U.S. Food and Drug Administration.
|
| 12 |
The synergy of the XPO1 inhibitors combined with the BET inhibitor INCB057643 in high-grade B-cell lymphoma via downregulation of MYC expression. Sci Rep. 2023 Oct 29;13(1):18554. doi: 10.1038/s41598-023-45721-z.
|
| 13 |
Pre-clinical evaluation of the MDM2-p53 antagonist RG7388 alone and in combination with chemotherapy in neuroblastoma. Oncotarget. 2015 Apr 30;6(12):10207-21.
|
| 14 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Target id: 1799).
|
|
|
|
|
|
|