Details of the Drug
General Information of Drug (ID: DMBD4K3)
| Drug Name |
Selinexor
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms | Xpovio; KPT 330; KPT-330; KPT-330(Selinexor); KPT330;Selinexor; 1393477-72-9; 31TZ62FO8F; CHEMBL3545185; SCHEMBL14678327; Selinexor; Selinexor (KPT-330); Selinexor [USAN:INN]; Tube706; UNII-31TZ62FO8F | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Indication |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ATC Code | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure |
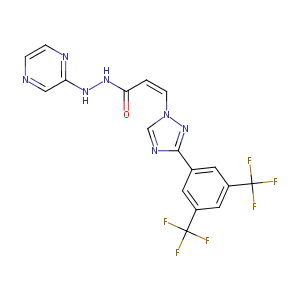 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 443.3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 5 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 12 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cross-matching ID | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Selinexor (Comorbidity)
|
|||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | ||||
|---|---|---|---|---|---|
| 2 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | ||||
| 3 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | ||||
| 4 | ClinicalTrials.gov (NCT04349098) Evaluation of Activity and Safety of Oral Selinexor in Participants With Severe COVID-19 Infection. U.S. National Institutes of Health. | ||||
| 5 | ClinicalTrials.gov (NCT02025985) Phase II Study of KPT-330 (Selinexor) in Female Patients With Advanced Gynaecologic Malignancies. U.S. National Institutes of Health. | ||||
| 6 | Inhibition of CRM1-dependent nuclear export sensitizes malignant cells to cytotoxic and targeted agents. Semin Cancer Biol. 2014 August; 0: 62-73. | ||||
| 7 | FDA label of Selinexor. The 2020 official website of the U.S. Food and Drug Administration. | ||||
| 8 | The synergy of the XPO1 inhibitors combined with the BET inhibitor INCB057643 in high-grade B-cell lymphoma via downregulation of MYC expression. Sci Rep. 2023 Oct 29;13(1):18554. doi: 10.1038/s41598-023-45721-z. | ||||
| 9 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 10 | Product Information. Ocrevus (ocrelizumab). Genentech, South San Francisco, CA. | ||||
| 11 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
