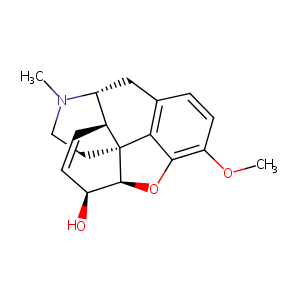
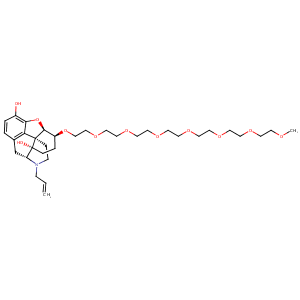
| 1 |
ClinicalTrials.gov (NCT02737059) Effect of Naloxegol on Gastric, Small Bowel, and Colonic Transit in Healthy Subjects
|
| 2 |
Codeine FDA Label
|
| 3 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 1673).
|
| 4 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7539).
|
| 5 |
Butorphanol: effects of a prototypical agonist-antagonist analgesic on kappa-opioid receptors. J Pharmacol Sci. 2005 Jun;98(2):109-16.
|
| 6 |
Association of Organic Cation Transporter 1 With Intolerance to Metformin in Type 2 Diabetes: A GoDARTS Study. Diabetes. 2015 May;64(5):1786-93.
|
| 7 |
Drug-drug interactions for UDP-glucuronosyltransferase substrates: a pharmacokinetic explanation for typically observed low exposure (AUCi/AUC) ratios. Drug Metab Dispos. 2004 Nov;32(11):1201-8.
|
| 8 |
Evaluation of 3'-azido-3'-deoxythymidine, morphine, and codeine as probe substrates for UDP-glucuronosyltransferase 2B7 (UGT2B7) in human liver microsomes: specificity and influence of the UGT2B7*2 polymorphism. Drug Metab Dispos. 2003 Sep;31(9):1125-33.
|
| 9 |
Polymorphism of human cytochrome P450 2D6 and its clinical significance: Part I. Clin Pharmacokinet. 2009;48(11):689-723.
|
| 10 |
Summary of information on human CYP enzymes: human P450 metabolism data. Drug Metab Rev. 2002 Feb-May;34(1-2):83-448.
|
| 11 |
Human UGT2B7 catalyzes morphine glucuronidation. Drug Metab Dispos. 1997 Jan;25(1):1-4.
|
| 12 |
Methadone inhibits CYP2D6 and UGT2B7/2B4 in vivo: a study using codeine in methadone- and buprenorphine-maintained subjects. Br J Clin Pharmacol. 2012 May;73(5):786-94.
|
| 13 |
Codeine induces human mast cell chemokine and cytokine production: involvement of G-protein activation. Allergy. 2007 May;62(5):532-8. doi: 10.1111/j.1398-9995.2007.01345.x.
|
| 14 |
Effects of common antitussive drugs on the hERG potassium channel current. J Cardiovasc Pharmacol. 2008 Dec;52(6):494-9. doi: 10.1097/FJC.0b013e31818eec8d.
|
| 15 |
Contribution of human esterases to the metabolism of selected drugs of abuse. Toxicol Lett. 2015 Jan 5;232(1):159-66. doi: 10.1016/j.toxlet.2014.10.026. Epub 2014 Oct 24.
|
| 16 |
2014 FDA drug approvals. Nat Rev Drug Discov. 2015 Feb;14(2):77-81.
|
| 17 |
Tarascon Pocket Pharmacopoeia 2018 Classic Shirt-Pocket Edition.
|
| 18 |
Naloxegol: treatment for opioid-induced constipation in chronic non-cancer pain. Ann Pharmacother. 2015 Mar;49(3):360-5.
|
| 19 |
ClinicalTrials.gov (NCT05770960) Colonic Motor Patterns in Healthy Volunteers
|
|
|
|
|
|
|