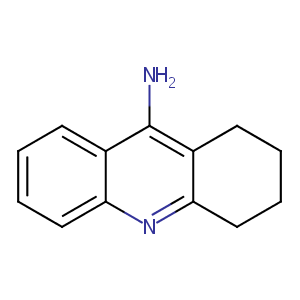
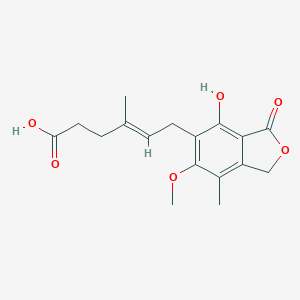
| 1 |
Recurrent recessive mutation in deoxyguanosine kinase causes idiopathic noncirrhotic portal hypertension.Hepatology. 2016 Jun;63(6):1977-86. doi: 10.1002/hep.28499. Epub 2016 Mar 31.
|
| 2 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6687).
|
| 3 |
Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015
|
| 4 |
A standard database for drug repositioning. Sci Data. 2017 Mar 14;4:170029.
|
| 5 |
Early termination of a trial of mycophenolate mofetil for treatment of interstitial cystitis/painful bladder syndrome: lessons learned. J Urol. 2011 Mar;185(3):901-6.
|
| 6 |
New conjugates of mycophenolic acid and their antiproliferative activity. J Asian Nat Prod Res. 2016 Nov;18(11):1057-62.
|
| 7 |
2019 Update of the Joint European League Against Rheumatism and European Renal Association-European Dialysis and Transplant Association (EULAR/ERA-EDTA) recommendations for the management of lupus nephritis. Ann Rheum Dis. 2020 Jun;79(6):713-723.
|
| 8 |
Mycophenolic Acid overcomes imatinib and nilotinib resistance of chronic myeloid leukemia cells by apoptosis or a senescent-like cell cycle arrest. Leuk Res Treatment. 2012;2012:861301.
|
| 9 |
Thiotepa, Busulfan, and Fludarabine Conditioning Regimen in T Cell-Replete HLA-Haploidentical Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Transplant. 2019 Jul;25(7):1407-1415.
|
| 10 |
Systemic Lupus Erythematosus Management in Pregnancy. Int J Womens Health. 2022 Feb 15;14:199-211.
|
| 11 |
Identification of potential drugs for diffuse large b-cell lymphoma based on bioinformatics and Connectivity Map database. Pathol Res Pract. 2018 Nov;214(11):1854-1867.
|
| 12 |
Associations between immune-suppressive and stimulating drugs and novel COVID-19-a systematic review of current evidence. Ecancermedicalscience. 2020 Mar 27;14:1022.
|
| 13 |
SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol. 2016 Aug;14(8):523-34.
|
| 14 |
Evidence that the clinical effects of cholinesterase inhibitors are related to potency and targeting of action. Int J Clin Pract Suppl. 2002 Jun;(127):6-19.
|
| 15 |
Tacrine sinusoidal uptake and biliary excretion in sandwich-cultured primary rat hepatocytes. J Pharm Pharm Sci. 2014;17(3):427-38.
|
| 16 |
Synthetic and natural compounds that interact with human cytochrome P450 1A2 and implications in drug development. Curr Med Chem. 2009;16(31):4066-218.
|
| 17 |
Combined glutathione-S-transferase M1 and T1 genetic polymorphism and tacrine hepatotoxicity. Clin Pharmacol Ther. 2000 Apr;67(4):432-7.
|
| 18 |
Comparative effects of cationic triarylmethane, phenoxazine and phenothiazine dyes on horse serum butyrylcholinesterase. Arch Biochem Biophys. 2008 Oct 15;478(2):201-5.
|
| 19 |
Inhibition of human carboxylesterases hCE1 and hiCE by cholinesterase inhibitors. Chem Biol Interact. 2013 Mar 25;203(1):226-30.
|
| 20 |
Reduction and scavenging of chemically reactive drug metabolites by NAD(P)H:quinone oxidoreductase 1 and NRH:quinone oxidoreductase 2 and variability in hepatic concentrations. Chem Res Toxicol. 2018 Feb 19;31(2):116-126.
|
| 21 |
Evaluating the Role of Multidrug Resistance Protein 3 (MDR3) Inhibition in Predicting Drug-Induced Liver Injury Using 125 Pharmaceuticals. Chem Res Toxicol. 2017 May 15;30(5):1219-1229. doi: 10.1021/acs.chemrestox.7b00048. Epub 2017 May 4.
|
| 22 |
Correlation of brain levels of 9-amino-1,2,3,4-tetrahydroacridine (THA) with neurochemical and behavioral changes. Eur J Pharmacol. 1989 Nov 28;173(1):53-64. doi: 10.1016/0014-2999(89)90008-3.
|
| 23 |
Crystal structure of human carboxylesterase 1 complexed with the Alzheimer's drug tacrine: from binding promiscuity to selective inhibition. Chem Biol. 2003 Apr;10(4):341-9. doi: 10.1016/s1074-5521(03)00071-1.
|
| 24 |
High-content imaging-based BAC-GFP toxicity pathway reporters to assess chemical adversity liabilities. Arch Toxicol. 2017 Mar;91(3):1367-1383. doi: 10.1007/s00204-016-1781-0. Epub 2016 Jun 29.
|
| 25 |
Refining the human iPSC-cardiomyocyte arrhythmic risk assessment model. Toxicol Sci. 2013 Dec;136(2):581-94. doi: 10.1093/toxsci/kft205. Epub 2013 Sep 19.
|
| 26 |
ADReCS-Target: target profiles for aiding drug safety research and application. Nucleic Acids Res. 2018 Jan 4;46(D1):D911-D917. doi: 10.1093/nar/gkx899.
|
| 27 |
Development of a highly sensitive cytotoxicity assay system for CYP3A4-mediated metabolic activation. Drug Metab Dispos. 2011 Aug;39(8):1388-95. doi: 10.1124/dmd.110.037077. Epub 2011 May 3.
|
| 28 |
C-440T/T-331C polymorphisms in the UGT1A9 gene affect the pharmacokinetics of mycophenolic acid in kidney transplantation. Pharmacogenomics. 2007 Sep;8(9):1127-41.
|
| 29 |
Interaction and transport characteristics of mycophenolic acid and its glucuronide via human organic anion transporters hOAT1 and hOAT3. Biochem Pharmacol. 2007 Jun 30;74(1):161-8.
|
| 30 |
Diabetes mellitus reduces activity of human UDP-glucuronosyltransferase 2B7 in liver and kidney leading to decreased formation of mycophenolic acid acyl-glucuronide metabolite. Drug Metab Dispos. 2011 Mar;39(3):448-55.
|
|
|
|
|
|
|