Details of the Drug
General Information of Drug (ID: DMRBMAU)
| Drug Name |
Mycophenolic acid
|
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Micofenolico acido; Micofenolico acido [Spanish]; Mycophenoic acid; Mycophenolate; Mycophenolsaeure; Myfortic; mycophenolic acid; Acide mycophenolique; Acido micofenolico; Acido micofenolico [INN-Spanish]; Acidum mycophenolicum; Lilly-68618; Ly 68618; Melbex; (4E)-6-(4-hydroxy-6-methoxy-7-methyl-3-oxo-1,3-dihydro-2-benzofuran-5-yl)-4-methylhex-4-enoic acid; 24280-93-1; 6-(4-Hydroxy-6-methoxy-7-methyl-3-oxo-5-phthalanyl)-4-methyl-4-hexenoic acid; CCRIS 5565; NSC 129185; NSC-129185; UNII-HU9DX48N0T
|
|||||||||||||||
| Indication |
|
|||||||||||||||
| Affected Organisms |
Humans and other mammals
|
|||||||||||||||
| Structure |
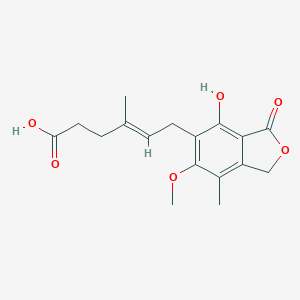 |
|||||||||||||||
| 3D MOL | 2D MOL | |||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 320.3 | ||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3.2 | |||||||||||||||
| Rotatable Bond Count (rotbonds) | 6 | |||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | |||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 6 | |||||||||||||||
| Chemical Identifiers |
|
|||||||||||||||
| Cross-matching ID | ||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | |||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | |||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Transporter (DTP) |
|
|||||||||||||||||||||||||||||||
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
Experimental Cancer Drug Sensitivity Information
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Mycophenolic acid (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
| 1 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | ||||
|---|---|---|---|---|---|
| 2 | A standard database for drug repositioning. Sci Data. 2017 Mar 14;4:170029. | ||||
| 3 | Early termination of a trial of mycophenolate mofetil for treatment of interstitial cystitis/painful bladder syndrome: lessons learned. J Urol. 2011 Mar;185(3):901-6. | ||||
| 4 | New conjugates of mycophenolic acid and their antiproliferative activity. J Asian Nat Prod Res. 2016 Nov;18(11):1057-62. | ||||
| 5 | 2019 Update of the Joint European League Against Rheumatism and European Renal Association-European Dialysis and Transplant Association (EULAR/ERA-EDTA) recommendations for the management of lupus nephritis. Ann Rheum Dis. 2020 Jun;79(6):713-723. | ||||
| 6 | Mycophenolic Acid overcomes imatinib and nilotinib resistance of chronic myeloid leukemia cells by apoptosis or a senescent-like cell cycle arrest. Leuk Res Treatment. 2012;2012:861301. | ||||
| 7 | Thiotepa, Busulfan, and Fludarabine Conditioning Regimen in T Cell-Replete HLA-Haploidentical Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Transplant. 2019 Jul;25(7):1407-1415. | ||||
| 8 | Systemic Lupus Erythematosus Management in Pregnancy. Int J Womens Health. 2022 Feb 15;14:199-211. | ||||
| 9 | Identification of potential drugs for diffuse large b-cell lymphoma based on bioinformatics and Connectivity Map database. Pathol Res Pract. 2018 Nov;214(11):1854-1867. | ||||
| 10 | Associations between immune-suppressive and stimulating drugs and novel COVID-19-a systematic review of current evidence. Ecancermedicalscience. 2020 Mar 27;14:1022. | ||||
| 11 | SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol. 2016 Aug;14(8):523-34. | ||||
| 12 | C-440T/T-331C polymorphisms in the UGT1A9 gene affect the pharmacokinetics of mycophenolic acid in kidney transplantation. Pharmacogenomics. 2007 Sep;8(9):1127-41. | ||||
| 13 | Interaction and transport characteristics of mycophenolic acid and its glucuronide via human organic anion transporters hOAT1 and hOAT3. Biochem Pharmacol. 2007 Jun 30;74(1):161-8. | ||||
| 14 | Diabetes mellitus reduces activity of human UDP-glucuronosyltransferase 2B7 in liver and kidney leading to decreased formation of mycophenolic acid acyl-glucuronide metabolite. Drug Metab Dispos. 2011 Mar;39(3):448-55. | ||||
| 15 | Bullingham R, Shah J, Goldblum R, Schiff M "Effects of food and antacid on the pharmacokinetics of single doses of mycophenolate mofetil in rheumatoid arthritis patients." Br J Clin Pharmacol 41 (1996): 513-6. [PMID: 8799515] | ||||
| 16 | Product Information. CellCept (mycophenolate mofetil). Roche Laboratories, Nutley, NJ. | ||||
| 17 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 18 | Product Information. Arava (leflunomide). Hoechst Marion-Roussel Inc, Kansas City, MO. | ||||
| 19 | Product Information. Prolia (denosumab). Amgen USA, Thousand Oaks, CA. | ||||
| 20 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 21 | Canadian Pharmacists Association. | ||||
| 22 | Product Information. Vumerity (diroximel fumarate). Alkermes, Inc, Cambridge, MA. | ||||
| 23 | Product Information. Gilenya (fingolimod). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 24 | Product Information. Ocrevus (ocrelizumab). Genentech, South San Francisco, CA. | ||||
| 25 | Product Information. Arcalyst (rilonacept). Regeneron Pharmaceuticals Inc, Tarrytown, NY. | ||||
| 26 | Product Information. Cimzia (certolizumab). UCB Pharma Inc, Smyrna, GA. | ||||
| 27 | CDC. Centers for Disease Control and Prevention/ "Recommendations of the advisory committtee on immunization practices (ACIP): use of vaccines and immune globulins in persons with altered immunocompetence." MMWR Morb Mortal Wkly Rep 42(RR-04) (1993): 1-18. [PMID: 20300058] | ||||
