| 1 |
ClinicalTrials.gov (NCT02210325) Efficacy and Safety Study of Oral Solithromycin Compared to Intramuscular Ceftriaxone Plus Oral Azithromycin in the Treatment of Patients With Gonorrhea
|
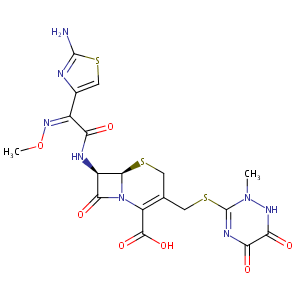
| 2 |
Ceftriaxone FDA Label
|
| 3 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 5326).
|
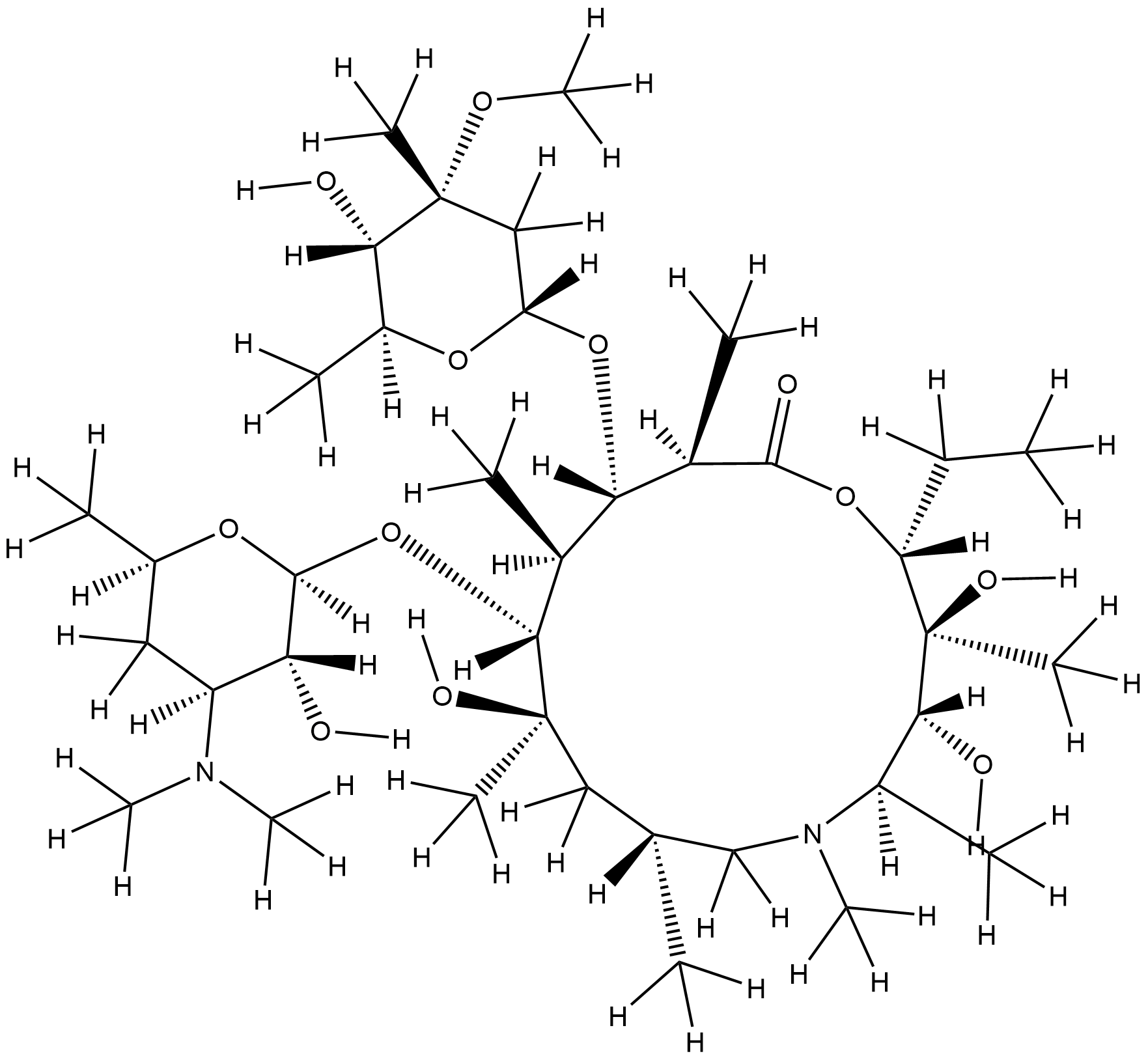
| 4 |
Azithromycin FDA Label
|
| 5 |
FDA Approved Drug Products from FDA Official Website. 2019. Application Number: (ANDA) 065511.
|
| 6 |
ClinicalTrials.gov (NCT04332107) Azithromycin for COVID-19 Treatment in Outpatients Nationwide. U.S. National Institutes of Health.
|
| 7 |
Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services.
|
| 8 |
Mammalian drug efflux transporters of the ATP binding cassette (ABC) family in multidrug resistance: A review of the past decade. Cancer Lett. 2016 Jan 1;370(1):153-64.
|
| 9 |
Intestinal transport of beta-lactam antibiotics: analysis of the affinity at the H+/peptide symporter (PEPT1), the uptake into Caco-2 cell monolayers and the transepithelial flux. Pharm Res. 1999 Jan;16(1):55-61.
|
| 10 |
Screening of antibiotics that interact with organic anion-transporting polypeptides 1B1 and 1B3 using fluorescent probes. Biol Pharm Bull. 2011;34(3):389-95.
|
| 11 |
Antibiotic resistance and production of extended-spectrum beta-lactamases amongst Klebsiella spp. from intensive care units in Europe. J Antimicrob Chemother. 1996 Sep;38(3):409-24.
|
| 12 |
Determination of the antibiotic resistance rates of Serratia marcescens isolates obtained from various clinical specimens. Niger J Clin Pract. 2019 Jan;22(1):125-130.
|
| 13 |
Drug-resistant genes carried by Acinetobacter baumanii isolated from patients with lower respiratory tract infection. Chin Med J (Engl). 2010 Sep;123(18):2571-5.
|
| 14 |
Investigation of the effects of some drugs and phenolic compounds on human dihydrofolate reductase activity. J Biochem Mol Toxicol. 2015 Mar;29(3):135-9.
|
| 15 |
ClinicalTrials.gov (NCT00035347) Intravenous Azithromycin Plus Intravenous Ceftriaxone Followed by Oral Azithromycin With Intravenous Levofloxacin Followed by Oral Levofloxacin for the Treatment of Moderate to Severely Ill Hospitalized Patients With Community Acquired Pneumonia
|
| 16 |
ClinicalTrials.gov (NCT01150747) Chlamydia Trachomatis Immunology and Vaccinology Study
|
|
|
|
|
|
|