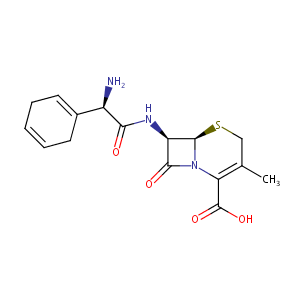
| Synonyms |
Anspor; Cefradin; Cefradina; Cefradinum; Cephradin; Cephradine; Eskacef; Sefril; Velosef; CEPHRADINE SODIUM; SKF D 39304; SQ 11436; VELOSEF 125; VELOSEF 250; VELOSEF 500; Anspor (TN); Cefradina [INN-Spanish]; Cefradinum [INN-Latin]; Cephradine (USP); Cephradine (anhydrous); Cephradine [USAN:BAN]; SQ-11436; SQ-22022; Velosef (TN); Cefradine (JAN/INN); SK&F D-39304; SK-D-39304; (6R,7R)-7-((R)-2-Amino-2-(1,4-cyclohexadien-1-yl)acetamido)-3-methyl-8-oxo-5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic acid; (6R,7R)-7-[[(2R)-2-amino-2-cyclohexa-1,4-dien-1-ylacetyl]amino]-3-methyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid; (6R,7R)-7-{[(2R)-2-amino-2-cyclohexa-1,4-dien-1-ylacetyl]amino}-3-methyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid; 7-(D-2-Amino-2-(1,4-cyclohexadien-1-yl)acetamido)-3-methyl-8-oxo-5-thia-1-azabicyclo(4.2.0)-oct-2-ene-2-caboxylic acid; 7-(D-2-Amino-2-(1,4-cyclohexadienyl)acetamide)desacetoxycephalosporanicacid; 7beta-[(2R)-2-(cyclohexa-1,4-dienyl)-2-phenylacetamido]-3-methyl-3,4-didehydrocepham-4-carboxylic acid
|