Details of the Drug
General Information of Drug (ID: DMUNSWV)
| Drug Name |
Cefradine
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Anspor; Cefradin; Cefradina; Cefradinum; Cephradin; Cephradine; Eskacef; Sefril; Velosef; CEPHRADINE SODIUM; SKF D 39304; SQ 11436; VELOSEF 125; VELOSEF 250; VELOSEF 500; Anspor (TN); Cefradina [INN-Spanish]; Cefradinum [INN-Latin]; Cephradine (USP); Cephradine (anhydrous); Cephradine [USAN:BAN]; SQ-11436; SQ-22022; Velosef (TN); Cefradine (JAN/INN); SK&F D-39304; SK-D-39304; (6R,7R)-7-((R)-2-Amino-2-(1,4-cyclohexadien-1-yl)acetamido)-3-methyl-8-oxo-5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic acid; (6R,7R)-7-[[(2R)-2-amino-2-cyclohexa-1,4-dien-1-ylacetyl]amino]-3-methyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid; (6R,7R)-7-{[(2R)-2-amino-2-cyclohexa-1,4-dien-1-ylacetyl]amino}-3-methyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid; 7-(D-2-Amino-2-(1,4-cyclohexadien-1-yl)acetamido)-3-methyl-8-oxo-5-thia-1-azabicyclo(4.2.0)-oct-2-ene-2-caboxylic acid; 7-(D-2-Amino-2-(1,4-cyclohexadienyl)acetamide)desacetoxycephalosporanicacid; 7beta-[(2R)-2-(cyclohexa-1,4-dienyl)-2-phenylacetamido]-3-methyl-3,4-didehydrocepham-4-carboxylic acid
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Indication |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Antibiotics
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ATC Code |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure |
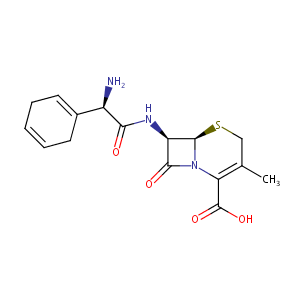 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 349.4 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 0.4 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 4 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 6 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ADMET Property |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cross-matching ID | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Transporter (DTP) |
|
|||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Cefradine (Comorbidity)
|
|||||||||||||||||||||||||||||||||||
References
