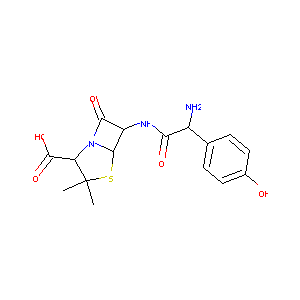
| Synonyms |
AMPC; Actimoxi; Amoclen; Amolin; Amopen; Amopenixin; Amoxi; Amoxibiotic; Amoxicaps; Amoxicilina; Amoxicillanyl; Amoxicilline; Amoxicillinum; Amoxiden; Amoxil; Amoxivet; Amoxycillin; Anemolin; Aspenil; Biomox; Bristamox; Cemoxin; Clamoxyl; Delacillin; DisperMox; Efpenix; Flemoxin; Hiconcil; Histocillin; Hydroxyampicillin; Ibiamox; Imacillin; Lamoxy; Larotid; Moxacin; Moxal; Moxatag; Ospamox; Pamoxicillin; Piramox; Polymox; Robamox; Sumox; Tolodina; Trimox; Unicillin; Utimox; Vetramox; Wymox; AMOXICILLIN CRYSTALLINE; AMOXICILLIN PEDIATRIC; Amoxicillin anhydrous; Amoxicilline [INN]; Amoxycillin Trihydrate; Metafarma capsules; Metifarma capsules; Sawamox PM; BLP 1410; AMK (TN); Actimoxi (TN); Alphamox (TN); Amoksibos (TN); Amoksiklav (TN); Amoxi-Mast; Amoxibiotic (TN); Amoxicilina (TN); Amoxicilina [INN-Spanish]; Amoxicillin (INN); Amoxicillin (TN); Amoxicillin (anhydrous); Amoxicilline [INN-French]; Amoxicillinum [INN-Latin]; Amoxiclav (TN); Amoxidal (TN); Amoxil (TN); Amoxin (TN); Apo-Amoxi; Augmentin (TN); BL-P 1410; BRL-2333; Bactox (TN); Betalaktam (TN); Cilamox (TN); Clamoxyl (TN); Curam (TN); D-Amoxicillin; Dedoxil (TN); Dispermox (TN); Duomox (TN); Enhancin (TN); Geramox (TN); Gimalxina (TN); Hiconcil (TN); Isimoxin (TN); Klavox (TN); Lamoxy (TN); Moxatag (TN); Moxilen (TN); Moxypen (TN); Moxyvit (TN); Nobactam (TN); Novamoxin (TN); Ospamox (TN); P-Hydroxyampicillin; Pamoxicillin (TN); Panamox (TN); Panklav (TN); Polymox (TN); Ro 10-8756; Samthongcillin (TN); Sandoz (TN); Senox (TN); Sinacilin (TN); Tolodina (TN); Trimox (TN); Wymox (TN); Yucla (TN); Zerrsox (TN); Zimox (TN); Apo-Amoxi (TN); Alpha-Amino-p-hydroxybenzylpenicillin; D-2-Amino-2-(4-hydroxyphenyl)acetamidopenicillanic acid; D-(-)-alpha-Amino-p-hydroxybenzylpenicillin; (-)-6-(2-Amino-2-(P-hydroxyphenyl)acetamido)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo-(3.2.0)heptane-2-carboxylic acid; (2S,5R,6R)-6-[[(2R)-2-amino-2-(4-hydroxyphenyl)acetyl]amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid; (2S,5R,6R)-6-{[(2R)-2-amino-2-(4-hydroxyphenyl)acetyl]amino}-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid; 4-Thia-1-azabicyclo(3.2.0)heptane-2-carboxylic acid, 6-(2-amino-2-(p-hydroxyphenyl)acetamido)-3,3-dimethyl-7-oxo-, D-(8CI); 6-(D-(-)-alpha-Amino-p-hydroxyphenylacetamido)penicillanic acid; 6-(D-(-)-p-Hydroxy-alpha-aminobenzyl)penicillin; 6-(p-Hydroxy-alpha-aminophenylacetamido)penicillanic acid; 6beta-[(2R)-2-amino-2-(4-hydroxyphenyl)acetamido]-2,2-dimethylpenam-3alpha-carbonyl; 6beta-[(2R)-2-amino-2-(4-hydroxyphenyl)acetamido]-2,2-dimethylpenam-3alpha-carboxylic acid
|