Details of the Drug
General Information of Drug (ID: DM0HPWY)
| Drug Name |
biochanin A
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Biochanin A; 491-80-5; Biochanin; 4'-Methylgenistein; 5,7-Dihydroxy-4'-methoxyisoflavone; 5,7-Dihydroxy-3-(4-methoxyphenyl)-4H-chromen-4-one; Genistein 4-methyl ether; 5,7-Dihydrox -4'-methoxyisoflavone; Biochanine A; 4H-1-Benzopyran-4-one, 5,7-dihydroxy-3-(4-methoxyphenyl)-; olmelin; Pratensol; NSC 123538; Biochanin-A; 5,7-dihydroxy-3-(4-methoxyphenyl)chromen-4-one; 4-Methylgenistein; C16H12O5; UNII-U13J6U390T; CCRIS 5449; 5,7-Dihydroxy-3-p-methoxyphenyl-4H-chromen-4-one; EINECS 207-744-7; NSC123538; Genistein 4'-methyl ether; 4'-methylgenistein; BIOCHANIN
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
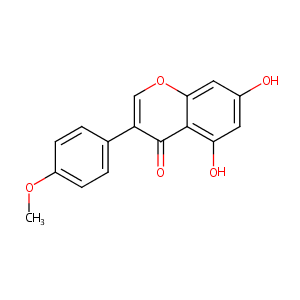 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 284.26 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 5 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Discovery agent | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | N.A. | |||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||
References
