Details of the Drug
General Information of Drug (ID: DM1D8XY)
| Drug Name |
Dantrolene
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Dantrium; Dantroleno; Dantrolenum; DANTROLENE SODIUM; Dantrium Intravenous; Dantrium (TN); Dantrolen (TN); Dantroleno [INN-Spanish]; Dantrolenum [INN-Latin]; F-368; Dantrolene (USAN/INN); Dantrolene [USAN:BAN:INN]; 1-(((5-(4-Nitrophenyl)-2-furanyl)methylene)amino)-2,4-imidazolidinedione; 1-((5-(p-Nitrophenyl)furfurylidene)amino)hydantoin; 1-({(1E)-[5-(4-nitrophenyl)furan-2-yl]methylidene}amino)imidazolidine-2,4-dione; 1-({[5-(4-nitrophenyl)furan-2-yl]methylidene}amino)imidazolidine-2,4-dione; 1-[(E)-[5-(4-nitrophenyl)furan-2-yl]methylideneamino]imidazolidine-2,4-dione
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Muscle Relaxants
|
||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
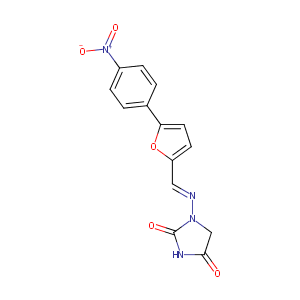 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 314.25 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 1.7 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 6 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Dantrolene (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 4172). | ||||
|---|---|---|---|---|---|
| 2 | BDDCS applied to over 900 drugs | ||||
| 3 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 4 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 5 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. | ||||
| 6 | Summary of information on human CYP enzymes: human P450 metabolism data. Drug Metab Rev. 2002 Feb-May;34(1-2):83-448. | ||||
| 7 | Effect of common medications on the expression of SARS-CoV-2 entry receptors in liver tissue. Arch Toxicol. 2020 Dec;94(12):4037-4041. doi: 10.1007/s00204-020-02869-1. Epub 2020 Aug 17. | ||||
| 8 | Insulin-like growth factors (IGF) I and II utilize different calcium signaling pathways in a primary human parathyroid cell culture model. World J Surg. 2006 Mar;30(3):333-45. doi: 10.1007/s00268-005-0339-8. | ||||
| 9 | Refining the human iPSC-cardiomyocyte arrhythmic risk assessment model. Toxicol Sci. 2013 Dec;136(2):581-94. doi: 10.1093/toxsci/kft205. Epub 2013 Sep 19. | ||||
| 10 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 11 | US Food and Drug Administration "FDA warns about serious risks and death when combining opioid pain or cough medicines with benzodiazepines requires its strongest warning.". | ||||
| 12 | Product Information. Sirturo (bedaquiline). Janssen Pharmaceuticals, Titusville, NJ. | ||||
| 13 | Product Information. Turalio (pexidartinib). Daiichi Sankyo, Inc., Parsippany, NJ. | ||||
| 14 | Product Information. Dantrium (dantrolene). Procter and Gamble Pharmaceutic, Cincinnati, OH. | ||||
| 15 | US Food and Drug Administration "FDA warns about serious risks and death when combining opioid pain or cough medicines with benzodiazepines requires its strongest warning.". | ||||
| 16 | Fuhr U, Maier-Bruggemann A, Blume H, et al. "Grapefruit juice increases oral nimodipine bioavailability." Int J Clin Pharmacol Ther 36 (1998): 126-32. [PMID: 9562227] | ||||
| 17 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 18 | Product Information. Adcetris (brentuximab vedotin). Seattle Genetics Inc, Bothell, WA. | ||||
| 19 | Product Information. Kynamro (mipomersen). Genzyme Corporation, Cambridge, MA. | ||||
| 20 | Canadian Pharmacists Association. | ||||
| 21 | Product Information. Juxtapid (lomitapide). Aegerion Pharmaceuticals Inc, Cambridge, MA. | ||||
| 22 | Al-Nawakil C, Willems L, Mauprivez C, et.al "Successful treatment of l-asparaginase-induced severe acute hepatotoxicity using mitochondrial cofactors." Leuk Lymphoma 55 (2014): 1670-4. [PMID: 24090500] | ||||
| 23 | Product Information. Zydelig (idelalisib). Gilead Sciences, Foster City, CA. | ||||
| 24 | Product Information. Zulresso (brexanolone). Sage Therapeutics, Inc., Cambridge, MA. | ||||
| 25 | Product Information. Reyvow (lasmiditan). Lilly, Eli and Company, Indianapolis, IN. | ||||
| 26 | Product Information. Addyi (flibanserin). Sprout Pharmaceuticals, Raleigh, NC. | ||||
