Details of the Drug
General Information of Drug (ID: DMG3F94)
| Drug Name |
Mestranol
|
||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Devocin; Menophase; Mestranolo; Mestranolum; Norquen; Ovastol; Component of Norinyl; Component of Norquen; Component of Ovulen; Ethynylestradiol methyl ether; Mestranol [Steroidal oestrogens]; Mestranolo [DCIT]; EE3ME; Ethinyl Estradiol 3 Methyl Ether; SC 4725; Component of Ortho-Novum; Delta-MVE; EE(sub3)ME; EthinylEstradiol 3-Methyl Ether; Ethinylestradiol 3-methyl ether; Ethinyloestradiol 3-methyl ether; Ethynylestradiol 3-methyl ether; Ethynyloestradiol 3-methyl ether; Inostral (steroid); Mestranolum [INN-Latin]; Mestranol (JP15/USP/INN); Mestranol [USAN:INN:BAN:JAN]; Alpha.-19-Norpregna-1,3,5(10)-trien-20-yn-17-ol, 3-meth; (17beta)-17-ethynyl-3-(methyloxy)estra-1,3,5(10)-trien-17-ol; 17-Ethynyl-3-methoxy-1,3,5(10)-oestratien-17-beta-ol; 17-Ethynyl-3-methoxyestra-1,3,5(10)-trien-17-ol; 17-Ethynylestradiol 3-methyl ether; 17-Ethynyloestradiol 3-methyl ether; 17-alpha-Ethinyl estradiol 3-methyl ether; 17-alpha-Ethinyl oestradiol 3-methyl ether; 17-alpha-Ethynyl-3-methoxy-1,3,5(10)-estratrien-17-beta-ol; 17-alpha-Ethynyloestradiol methyl ether; 17-ethynyl-3-methoxyestra-1(10),2,4-trien-17beta-ol; 17-ethynyl-3-methoxyoestra-1(10),2,4-trien-17beta-ol; 17alpha-Ethinyl estradiol 3-methyl ether; 17alpha-Ethinyl oestradiol 3-methyl ether; 17alpha-Ethinylestradiol 3-methyl ether; 17alpha-Ethynylestradiol 3-methyl ether; 17alpha-Ethynylestradiol methyl ether; 17alpha-Ethynyloestradiol 3-methyl ether; 17beta-Estradiol, 17-ethynyl-, 3-(methyl ether); 3-Methoxy-17-alpha-19-norpregna-1,3,5(10)-trien-20-yn-17-ol; 3-Methoxy-17-alpha-ethinylestradiol; 3-Methoxy-17-alpha-ethinyloestradiol; 3-Methoxy-17-alpha-ethynyl-1,3,5(10)-estratrien-17-beta-ol; 3-Methoxy-17-alpha-ethynyl-1,3,5(10)-oestratrien-17-beta-ol; 3-Methoxy-17-alpha-ethynylestradiol; 3-Methoxy-17-alpha-ethynyloestradiol; 3-Methoxy-17-ethynyloestradiol-17-beta; 3-Methoxy-17alpha-ethinylestradiol; 3-Methoxy-17alpha-ethinyloestradiol; 3-Methoxy-17alpha-ethynyl-1,3,5(10)-estratrien-17beta-ol; 3-Methoxy-17alpha-ethynyl-1,3,5(10)-oestratrien-17beta-ol; 3-Methoxy-17alpha-ethynylestradiol; 3-Methoxy-17alpha-ethynyloestradiol; 3-Methoxy-19-nor-17-alpha-pregna-1,3,5(10)-trien-20-yn-17-ol; 3-Methoxy-19-nor-17alpha-pregna-1,3,5(10)-trien-20-yn-17-ol; 3-Methoxy-19-norpregna-1,3,5(10)-trien-20-yn-17beta-ol; 3-Methoxyethynylestradiol; 3-Methoxyethynyloestradiol; 3-Methylethynylestradiol; 3-Methylethynyloestradiol; 3-O-Methylethynylestradiol
|
||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||
| Therapeutic Class |
Estrogens
|
||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||
| Structure |
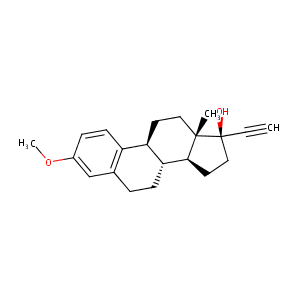 |
||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 310.4 | |||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 4 | ||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 2 | ||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 2 | ||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Contraception | |||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | QA21 | |||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Mestranol (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
