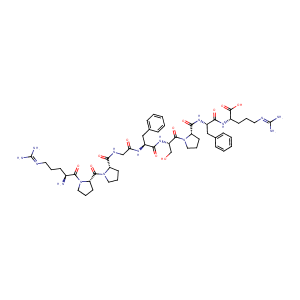
| Chemical Identifiers |
- Formula
- C50H73N15O11
- IUPAC Name
(2S)-2-[[(2S)-2-[[(2S)-1-[(2S)-2-[[(2S)-2-[[2-[[(2S)-1-[(2S)-1-[(2S)-2-amino-5-(diaminomethylideneamino)pentanoyl]pyrrolidine-2-carbonyl]pyrrolidine-2-carbonyl]amino]acetyl]amino]-3-phenylpropanoyl]amino]-3-hydroxypropanoyl]pyrrolidine-2-carbonyl]amino]-3-phenylpropanoyl]amino]-5-(diaminomethylideneamino)pentanoic acid - Canonical SMILES
-
C1C[C@H](N(C1)C(=O)[C@@H]2CCCN2C(=O)[C@H](CCCN=C(N)N)N)C(=O)NCC(=O)N[C@@H](CC3=CC=CC=C3)C(=O)N[C@@H](CO)C(=O)N4CCC[C@H]4C(=O)N[C@@H](CC5=CC=CC=C5)C(=O)N[C@@H](CCCN=C(N)N)C(=O)O
- InChI
-
InChI=1S/C50H73N15O11/c51-32(16-7-21-56-49(52)53)45(72)65-25-11-20-39(65)47(74)64-24-9-18-37(64)43(70)58-28-40(67)59-34(26-30-12-3-1-4-13-30)41(68)62-36(29-66)46(73)63-23-10-19-38(63)44(71)61-35(27-31-14-5-2-6-15-31)42(69)60-33(48(75)76)17-8-22-57-50(54)55/h1-6,12-15,32-39,66H,7-11,16-29,51H2,(H,58,70)(H,59,67)(H,60,69)(H,61,71)(H,62,68)(H,75,76)(H4,52,53,56)(H4,54,55,57)/t32-,33-,34-,35-,36-,37-,38-,39-/m0/s1
- InChIKey
-
QXZGBUJJYSLZLT-FDISYFBBSA-N
|