Details of the Drug
General Information of Drug (ID: DM6Y3A0)
| Drug Name |
GALLICACID
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Gallic acid; 3,4,5-Trihydroxybenzoic acid; 149-91-7; gallate; Benzoic acid, 3,4,5-trihydroxy-; Gallic acid, tech.; GALOP; Pyrogallol-5-carboxylic acid; Kyselina gallova; HSDB 2117; 3,4,5-Trihydroxybenzoate; CCRIS 5523; Kyselina gallova [Czech]; Gallic acid polymer; UNII-632XD903SP; NSC 674319; Gallicum acidum; CHEBI:30778; AI3-16412; Kyselina 3,4,5-trihydroxybenzoova; EINECS 205-749-9; NSC 20103; BRN 2050274; GALLIC ACID ANHYDROUS; Kyselina 3,4,5-trihydroxybenzoova [Czech]; CHEMBL288114; LNTHITQWFMADLM-UHFFFAOYSA-N; 632XD903SP
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
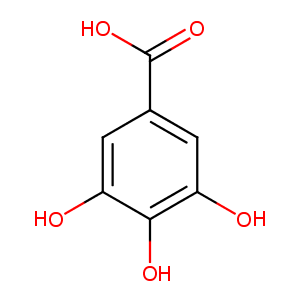 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 170.12 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 0.7 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 4 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 5 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Discovery agent | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | N.A. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
