Details of the Drug
General Information of Drug (ID: DM82Y75)
| Drug Name |
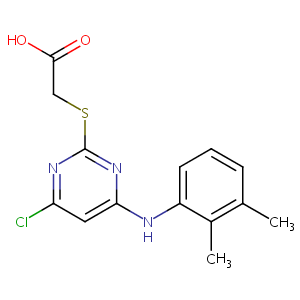
PIRINIXIC ACID
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Pirinixic acid; 50892-23-4; WY-14643; [4-Chloro-6-(2,3-xylidino)-2-pyrimidinylthio]acetic acid; WY-14,643; WY14643; Wyeth 14,643; Wy 14643; Pirinixic acid [INN]; WY-14643 (Pirinixic Acid); (4-Chloro-6-(2,3-xylidino)-2-pyrimidinylthio)acetic acid; UNII-86C4MRT55A; C14H14ClN3O2S; CCRIS 133; Acidum pirinixicum [INN-Latin]; Acide pirinixique [INN-French]; Acido pirinixico [INN-Spanish]; NSC310038; NSC-310038; NSC 310038; BRN 0759945; ((4-Chloro-6-((2,3-dimethylphenyl)amino)-2-pyrimidinyl)thio)acetic acid; Acetic acid, ((4-chloro-6-(
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 323.8 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 4.2 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 5 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 6 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Pulmonary fibrosis | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | CB03.4 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
