| Synonyms |
CEPHARANTHINE; Cepharanthin; (+)-Cepharanthine; O-Methylcepharanoline; Cepharantin; Cepharanthine [JAN]; UNII-7592YJ0J6T; CCRIS 6539; NSC 623442; BRN 0075231; 6',12'-Dimethoxy-2,2'-dimethyl-6,7-(methylenebis(oxy))oxyacanthan; CHEBI:3546; 7592YJ0J6T; NSC623442; Cepharanthine (JAN); NSC-623442; ecaene (non-preferred name); DSSTox_RID_81253; DSSTox_CID_25957; DSSTox_GSID_45957; Oxyacanthan, 6',12'-dimethoxy-2,2'-dimethyl-6,7-(methylenebis(oxy))-; CAS-481-49-2; SR-01000779734; Cepharanthin,(S)
|
| Chemical Identifiers |
- Formula
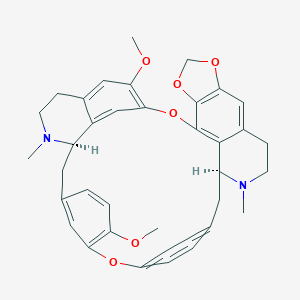
- C37H38N2O6
- IUPAC Name
(14S,27R)-22,33-dimethoxy-13,28-dimethyl-2,5,7,20-tetraoxa-13,28-diazaoctacyclo[25.6.2.216,19.13,10.121,25.04,8.031,35.014,39]nonatriaconta-1(33),3(39),4(8),9,16(38),17,19(37),21,23,25(36),31,34-dodecaene - Canonical SMILES
-
CN1CCC2=CC3=C(C4=C2C1CC5=CC=C(C=C5)OC6=C(C=CC(=C6)CC7C8=CC(=C(C=C8CCN7C)OC)O4)OC)OCO3
- InChI
-
InChI=1S/C37H38N2O6/c1-38-13-11-24-18-31(41-4)33-20-27(24)28(38)16-23-7-10-30(40-3)32(17-23)44-26-8-5-22(6-9-26)15-29-35-25(12-14-39(29)2)19-34-36(37(35)45-33)43-21-42-34/h5-10,17-20,28-29H,11-16,21H2,1-4H3/t28-,29+/m1/s1
- InChIKey
-
YVPXVXANRNDGTA-WDYNHAJCSA-N
|