Details of the Drug
General Information of Drug (ID: DMCH7RU)
| Drug Name |
Pheniramine
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Avil; Feniramina; Feniramine; Histapyridamine; Metron; Pheniraminum; Propheniramine; Prophenpyridamine; Pyriton; Trimeton; Tripoton; Pheniramine Bimaleate; Avil (TN); Feniramina [INN-Spanish]; P-Aminosalicylsaures salz; Pheniramine (INN); Pheniramine [INN:BAN]; Pheniraminum [INN-Latin]; PHENIRAMINE (SEE ALSO PHENIRAMINE MALEATE 132-20-7); Dimethyl(3-phenyl-3-(2-pyridyl)propyl)amine; N,N-Dimethyl-3-phenyl-3-(2-pyridyl)propylamine; N,N-dimethyl-3-phenyl-3-pyridin-2-ylpropan-1-amine; 1-Phenyl-1-(2-pyridyl)-3-dimethylaminopropane; 2-(3-Dimethylamino-1-phenylpropyl)pyridine; 2-(alpha-(2-Dimethylaminoethyl)benzyl)pyridine; 2-Pyridinepropanamine, N,N-dimethyl-gamma-phenyl-(9CI); 2-[.alpha.-(2-Dimethylaminoethyl)benzyl]pyridine; 2-[.alpha.-[2-(Dimethylamino)ethyl]benzyl]pyridine; 3-Phenyl-3-(2-pyridyl)-N,N-dimethylpropylamine; 3-Phenyl-3-(2-pyridyl)-N,N-dimethylpropylanine
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Indication |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Antiallergic Agents
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ATC Code |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure |
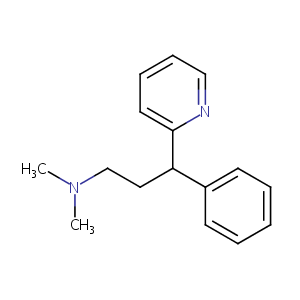 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 240.34 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.8 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 5 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ADMET Property |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cross-matching ID | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Allergic rhinitis | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | CA08.0 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
