Details of the Drug
General Information of Drug (ID: DMCP2KH)
| Drug Name |
HE2100
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Androstenediol; Hermaphrodiol; 5-Androstenediol; Androst-5-enediol; 521-17-5; Androst-5-ene-3beta,17beta-diol; Neumune; 3beta,17beta-Dihydroxyandrost-5-ene; delta(sup 5)-Androstenediol; Androstenediol [JAN]; UNII-95PS51EMXY; Androst-5-enediol (VAN); (3beta,17beta)-androst-5-ene-3,17-diol; 5-AED; 3beta,17beta-Dihydroxy-5-androstene; androst-5-en-3beta,17beta-diol; EINECS 208-306-8; NSC 12163; 95PS51EMXY; ANDROST-5-ENE-3-beta,17-beta-DIOL; CHEMBL440283; CHEBI:2710; (3-beta,17-beta)-Androst-5-ene-3,17-diol; delta(sup; ANDROSTENEDIOL
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
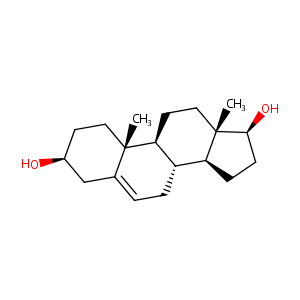 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 290.4 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3.5 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 0 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 2 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Thrombocytopenia | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 3B64 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
