Details of the Drug
General Information of Drug (ID: DMEGIQ6)
| Drug Name |
Nitrazepam
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Alodorm; Apodorm; Benzalin; Calsamin; Calsmin; Cerson; Dormalon; Dumolid; Eatan; Epibenzalin; Epinelbon; Eunoctin; Eunoktin; Gerson; Hipnax; Hipsal; Ibrovek; Imadorm; Imeson; Imesont; Ipersed; Magadon; Megadon; Mitidin; Mogadan; Mogadon; Mogadone; Nelbon; Nelmat; Neozepam; Neuchlonic; Nitrados; Nitravet; Nitrazadon; Nitrazep; Nitrazepamum; Nitrempax; Nitrenpax; Nitrodiazepam; Noctesed; Novanox; Pacisyn; Paxisyn; Pelson; Persopit; Radedorm; Relact; Remnos; Serenade; Somitran; Somnased; Somnibel; Somnite; Sonebon; Sonnolin; Surem; Trazenin; Unisomnia; Aliud Brand of Nitrazepam; Allphar Brand of Nitrazepam; Alphapharma Brand of Nitrazepam; Alpharma Brand of Nitrazepam; Alter Brand of Nitrazepam; CSP Brand of Nitrazepam; DDSA Brand of Nitrazepam; Dermatech Brand of Nitrazepam; Desitin Brand of Nitrazepam; Dormicum Brand of Nitrazepam; Eatan N; ICN Brand of Nitrazepam; Neuraxpharm Brand of Nitrazepam; Nitrazepam AL; Norgine Brand of Nitrazepam; Pfleger Brand of Nitrazepam; Protea Brand of Nitrazepam; Rhoxalpharma Brand of Nitrazepam; Sandoz nitrazepam; Scheurich Brand of Nitrazepam; Sonebon Tofraniln A; Taurus Brandof Nitrazepam; United Drug Brand of Nitrazepam; Wernigerode Brand of Nitrazepam; LA 1; S 2000; Alodorm (TN); Apo-nitrazepam tablets BP; Apodorm (TN); Arem (TN); Benzalin (TN); Cavodan (TN); Ct-Arzneimittel Brand of Nitrazepam; Dima (TN); Dormalon (TN); Dormicum (anticonvulsant); Dormigen (TN); Dormin-5; Dormo-Puren; Dumolid (TN); Eatan N (TN); Eunoctin (TN); Hypnotex (TN); ISOPROPYLACETATE/NITRAZEPAM; Imeson (TN); Insoma (TN); Insomin (TN); Ipersed (TN); LA 1 (VAN); Mitidin (TN); Mogadan (TN); Mogadon (TN); N-Desmethylnimetazepam; Nitavan (TN); Nitepam (TN); Nitrados (TN); Nitrapan (TN); Nitravet (TN); Nitrazadon (TN); Nitrazep (TN); Nitrazepam (TN); Nitrazepam Capsules BP 1993 (TN); Nitrazepam Oral Suspension BP 1993 (TN); Nitrazepam Tablets BP 1993 (TN); Nitrazepam-10; Nitrazepam-5; Nitrazepam-neuraxpharm; Nitrazepamum [INN-Latin]; Nitrazepan (TN); Nitrazepol (TN); Nitredon (TN); Nitrosun (TN); Novanox (TN); Numbon (TN); Onirema (TN); Ormodon (TN); Pacisyn (TN); Paxadorm (TN); Pelson (TN); Pelsonfilina (TN); Protraz (TN); Radedorm (TN); Remnos (TN); Rhoxal-nitrazepam; Ro 4-5360; Ro 5-3059; Ro 53-60; Serenade (TN); Somnibel N (TN); Somnipar (TN); Somnite (TN); Sonebon (TN); Sonotrat (TN); Surem (TN); Tri (TN); Unisomnia (TN); Dormo-Puren (TN); Nitrazepam (JP15/USAN/INN); Nitrazepam [USAN:INN:BAN:JAN]; 1,3-Dihydro-7-nitro-5-phenyl-2H-1,4-benzodiazepin-2-one; 2,3-Dihydro-7-nitro-5-phenyl-1H-1,4-benzodiazepin-2-on; 7-Nitro-1,3-dihydro-5-phenyl-2H-1,4-benzodiazepin-2-one; 7-Nitro-5-phenyl-1,3-dihydro-2H-1,4-benzodiazepin-2-one; 7-Nitro-5-phenyl-2,3-dihydro-1H-1,4-benzodiazepin-2-one; 7-nitro-5-phenyl-1,3-dihydro-1,4-benzodiazepin-2-one
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Therapeutic Class |
Antianxiety Agents
|
||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
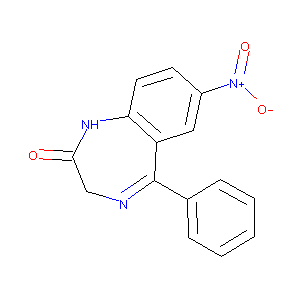 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 281.27 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.2 | ||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 1 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 4 | ||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
