Details of the Drug
General Information of Drug (ID: DMJQDUC)
| Drug Name |
Aminoguanidine
|
||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
aminoguanidine; Pimagedine; Hydrazinecarboximidamide; Guanyl hydrazine; Monoaminoguanidine; 2-aminoguanidine; 79-17-4; Imino semicarbazide; Aminate base; 2-azanylguanidine; Pimagedine [INN]; GUANIDINE, AMINO-; 1-aminoguanidine; UNII-SCQ4EZQ113; Hydrazinecarboximidamide(9CI); CCRIS 3511; EINECS 201-183-1; Aminoguanidine, Hemisulfate; SCQ4EZQ113; CHEMBL225304; CHEBI:40618; HAMNKKUPIHEESI-UHFFFAOYSA-N; guanylhydrazine; GER-11; AGU; amino guanidine; 1-amino-guanidine; Aminoguanidine (AG); Tocris-0787; Carbonohydrazonic diamide; INCB3284
|
||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||
| Structure |
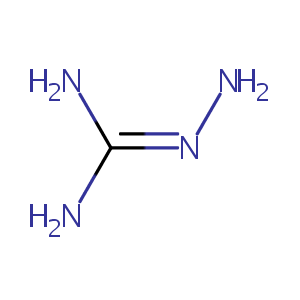 |
||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 74.09 | |||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -1.5 | ||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 0 | ||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 3 | ||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 2 | ||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| ICD Disease Classification | 15 Disease of the musculoskeletal system/connective tissue | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Disease Class | ICD-11: FA20 Rheumatoid arthritis | |||||||||||||||||||||||
| The Studied Tissue | Synovial tissue | |||||||||||||||||||||||
| The Studied Disease | Rheumatoid arthritis [ICD-11:FA20] | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
