Details of the Drug
General Information of Drug (ID: DMLN2GQ)
| Drug Name |
Oxalic Acid
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
ethanedioic acid; 144-62-7; Aktisal; Aquisal; Oxiric acid; Oxalsaeure; Oxaalzuur; Kyselina stavelova; Acide oxalique; Acido ossalico; Acidum oxalicum; Caswell No. 625; Oxaalzuur [Dutch]; Oxalsaeure [German]; NCI-C55209; Ethanedionic acid; Acide oxalique [French]; Acido ossalico [Italian]; Kyselina stavelova [Czech]; CCRIS 1454; EPA Pesticide Chemical Code 009601; Ethane-1,2-dioic acid; HSDB 1100; AI3-26463; NSC 62774; UNII-9E7R5L6H31; BRN 0385686; HOOCCOOH; Oxalic acid anhydrous; C2H2O4; EINECS 205-634-3; CHEBI:16995
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
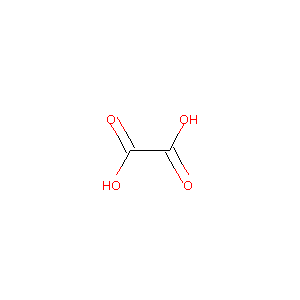 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 90.03 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -0.3 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 4 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Discovery agent | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | N.A. | |||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||
References
