Details of the Drug
General Information of Drug (ID: DMO50U2)
| Drug Name |
Naratriptan
|
||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Colatan; Naramig; Naratriptanum; Amerge (TN); Naramig (TN); Naratriptan (INN); Amerge, Naramig,Naratriptan; N-methyl-2-(3-(1-methylpiperiden-4-yl)indole-5-yl)ethanesulfonamide; N-methyl-2-[3-(1-methylpiperidin-4-yl)-1H-indol-5-yl]ethanesulfonamide; N-methyl-2-[3-(1-methyl-4-piperidyl)-1H-indol-5-yl]-ethanesulfonamide
|
||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||
| Therapeutic Class |
Vasoconstrictor Agents
|
||||||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||
| Structure |
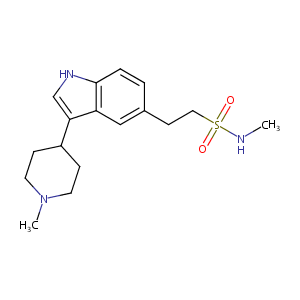 |
||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 335.5 | |||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2 | ||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 5 | ||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 4 | ||||||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Transporter (DTP) |
|
||||||||||||||||||||||||||
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Headache | |||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 8A80-8A84 | |||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Naratriptan (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Naratriptan FDA Label | ||||
|---|---|---|---|---|---|
| 2 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 45). | ||||
| 3 | BDDCS applied to over 900 drugs | ||||
| 4 | Critical Evaluation of Human Oral Bioavailability for Pharmaceutical Drugs by Using Various Cheminformatics Approaches | ||||
| 5 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 6 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 7 | Triptans in pregnancy. Ther Drug Monit. 2008 Feb;30(1):5-9. | ||||
| 8 | OCT1 mediates hepatic uptake of sumatriptan and loss-of-function OCT1 polymorphisms affect sumatriptan pharmacokinetics. Clin Pharmacol Ther. 2016 Jun;99(6):633-41. | ||||
| 9 | Migraine pharmacotherapy with oral triptans: a rational approach to clinical management. Expert Opin Pharmacother. 2000 Mar;1(3):391-404. | ||||
| 10 | G protein beta3 polymorphism and triptan response in cluster headache. Clin Pharmacol Ther. 2007 Oct;82(4):396-401. doi: 10.1038/sj.clpt.6100159. Epub 2007 Mar 14. | ||||
| 11 | Chan BSH, Graudins A, Whyte IM, Dawson AH, Braitberg G, Duggin GG "Serotonin syndrome resulting from drug interactions." Med J Aust 169 (1998): 523-5. [PMID: 9861909] | ||||
| 12 | Vizcaychipi MP, Walker S, Palazzo M "Serotonin syndrome triggered by tramadol." Br J Anaesth 99 (2007): 919. [PMID: 18006535] | ||||
| 13 | Achamallah NS "Visual hallucinations after combining fluoxetine and dextromethorphan ." Am J Psychiatry 149 (1992): 1406. [PMID: 1530079] | ||||
| 14 | Product Information. Northera (droxidopa). Chelsea Therapeutics Inc, Charlotte, NC. | ||||
| 15 | Ciraulo DA, Shader RI "Fluoxetine drug-drug interactions: I. Antidepressants and antipsychotics." J Clin Psychopharmacol 10 (1990): 48-50. [PMID: 1968472] | ||||
| 16 | Alvine G, Black DW, Tsuang D "Case of delirium secondary to phenelzine/L-tryptophan combination." J Clin Psychiatry 51 (1990): 311. [PMID: 2365671] | ||||
| 17 | Product Information. Belviq (lorcaserin). Eisai Inc, Teaneck, NJ. | ||||
