Details of the Drug
General Information of Drug (ID: DMOL793)
| Drug Name |
Trifarotene
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Trifarotene; UNII-0J8RN2W0HK; 895542-09-3; CD5789; 0J8RN2W0HK; Trifarotene [USAN:INN]; CD 5789; SCHEMBL381493; GTPL9962; CHEMBL3707313; DTXSID30237781; EX-A2704; DB12808; compound 15b [PMID: 29706423]; 3''-Tert-butyl-4'-(2-hydroxyethoxy)-4''-(pyrrolidin-1-yl)(1,1':3',1'')terphenyl-4-carboxylic acid; HY-100256; CS-0018407; 4-[3-(3-tert-butyl-4-pyrrolidin-1-ylphenyl)-4-(2-hydroxyethoxy)phenyl]benzoic acid; [1,1':3',1''-Terphenyl]-4-carboxylic acid, 3''-(1,1-dimethylethyl)-4'-(2-hydroxyethoxy)-4''-(1-pyrrolidinyl)-
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
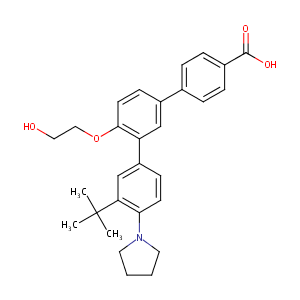 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 459.6 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 6.3 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 8 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 5 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Acne vulgaris | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | ED80 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health Human Services. 2019 | ||||
|---|---|---|---|---|---|
| 2 | FDA Approved Drugs: Aklief? | ||||
| 3 | Kosoglou T, Statkevich P, Johnson-Levonas AO, Paolini JF, Bergman AJ, Alton KB: Ezetimibe: a review of its metabolism, pharmacokinetics and drug interactions. Clin Pharmacokinet. 2005;44(5):467-94. | ||||
| 4 | FDA label of Trifarotene. The 2020 official website of the U.S. Food and Drug Administration. | ||||
