Details of the Drug
General Information of Drug (ID: DMQNOFA)
| Drug Name |
BAY11-7082
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
bay 11-7082; 19542-67-7; (E)-3-Tosylacrylonitrile; Bay 11-7821; (E)-3-(p-Toluenesulfonyl)acrylonitrile; BAY-11-7082; BAY11-7082; UNII-4Y5G2A4F6O; BAY-11-7821; (E)-3-(4-Methylphenyl)sulfonylprop-2-enenitrile; BAY-117082; BAY-117821; CHEMBL403183; 4Y5G2A4F6O; 3-(4-methylphenylsulfonyl)-2-propenenitrile; CHEBI:85928; 3-[(4-methylphenyl)sulfonyl]prop-2-enenitrile; AK129348; (E)-3-(4-Methylphenylsulfonyl)-2-propenenitrile; (E)3-[(4-Methylphenyl)sulfonyl]-2-propenenitrile; J-501956; (2E)-3-[(4-methylphenyl)sulfonyl]prop-2-enenitrile
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
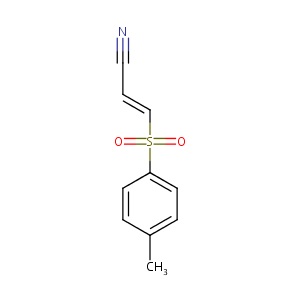 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 207.25 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 1.4 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
