Details of the Drug
General Information of Drug (ID: DMTCMH7)
| Drug Name |
1,4-Naphthoquinone
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
1,4-NAPHTHOQUINONE; 130-15-4; naphthalene-1,4-dione; p-Naphthoquinone; NAPHTHOQUINONE; alpha-Naphthoquinone; 1,4-Naphthylquinone; USAF CY-10; 1,4-dihydronaphthalene-1,4-dione; 1,4-Dihydro-1,4-diketonaphthalene; 1,4-Naftochinon; RCRA waste number U166; UNII-RBF5ZU7R7K; NSC 9583; 1,4-Naftochinon [Czech]; 1,4-Naphthaquinone; CCRIS 5424; HSDB 2037; EINECS 204-977-6; RBF5ZU7R7K; RCRA waste no U166; CHEMBL55934; AI3-24292; NQ-1; CHEBI:27418; NSC9583; FRASJONUBLZVQX-UHFFFAOYSA-N; MFCD00001676; 1,4-naphtho-quinone
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
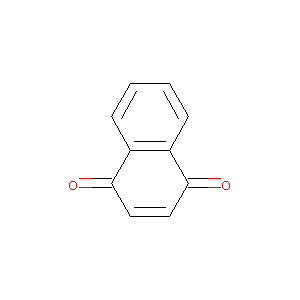 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 158.15 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 1.7 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 0 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 2 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
