Details of the Drug
General Information of Drug (ID: DMZ0Q1G)
| Drug Name |
Permethrin
|
||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Ambush; PerFoam; Transpermethrin; Acticin Cream; Elimite Cream; Nix Cream Rinse; Transpermethrin [ISO];FMC 35171; NRDC 146; NRDC 148; PS758_SUPELCO; Permethrin (isomers); Permethrin (isomers) solution; Permethrine,c&t; Trans-(+-)-Permethrin; [3-(phenyloxy)phenyl]methyl 3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropanecarboxylate; (+)-CIS-PERMETHRIN; (+)-trans-Permethrin; (+-)-cis-Fmc 33297; (+-)-cis-Permethrin; (+-)-trans-Permethrin; (3-Phenoxyphenyl)methyl (+-)-cis,trans-3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropanecarboxylate; (3-phenoxyphenyl)methyl 3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropane-1-carboxylate; 1RS,cis-Permethrin; 1RS-trans-Permethrin; 3-Phenoxybenzyle (1RS)-cis, trans-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropane carboxylate; 3-phenoxybenzyl 3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropanecarboxylate
|
||||||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Insecticides
|
||||||||||||||||||||||||||||||||||
| Affected Organisms |
Scabies (Sarcoptes scabei) and other insects
|
||||||||||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||||||
| Structure |
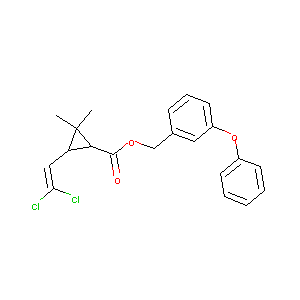 |
||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 391.3 | |||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 6.5 | ||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 7 | ||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | ||||||||||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Permethrin FDA Label | ||||
|---|---|---|---|---|---|
| 2 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | ||||
| 3 | Critical Evaluation of Human Oral Bioavailability for Pharmaceutical Drugs by Using Various Cheminformatics Approaches | ||||
| 4 | In vitro assays for repellents and deterrents for ticks: differing effects of products when tested with attractant or arrestment stimuli. Med Vet Entomol. 2003 Dec;17(4):370-8. | ||||
| 5 | Human metabolic interactions of environmental chemicals. J Biochem Mol Toxicol. 2007;21(4):182-6. | ||||
| 6 | Exposure to Insecticides Modifies Gene Expression and DNA Methylation in Hematopoietic Tissues In Vitro. Int J Mol Sci. 2023 Mar 26;24(7):6259. doi: 10.3390/ijms24076259. | ||||
| 7 | Pyrethroids: cytotoxicity and induction of CYP isoforms in human hepatocytes. Drug Metabol Drug Interact. 2008;23(3-4):211-36. | ||||
