| 1 |
ClinicalTrials.gov (NCT01919723) Ticagrelor and Eptifibatide Bolus-Only Versus Ticagrelor and Eptifibatide Bolus Plus Abbreviated Infusion
|
| 2 |
Ticagrelor-Induced Syncope/Bradyarrhythmia. Cureus. 2021 Jan 23;13(1):e12874.
|
| 3 |
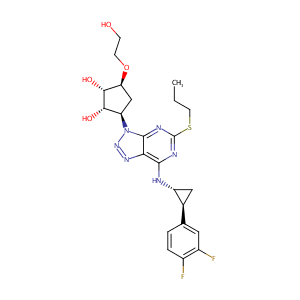
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 1765).
|
| 4 |
ClinicalTrials.gov (NCT01732822) A Study Comparing Cardiovascular Effects of Ticagrelor and Clopidogrel in Patients With Peripheral Artery Disease. U.S. National Institutes of Health.
|
| 5 |
Antithrombotic Therapy for Symptomatic Peripheral Arterial Disease: A Systematic Review and Network Meta-Analysis. Drugs. 2022 Aug;82(12):1287-1302.
|
| 6 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6585).
|
| 7 |
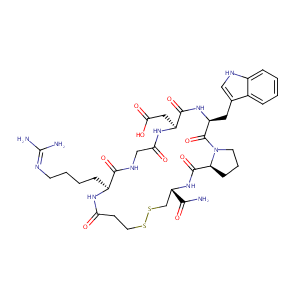
Eptifibatide FDA Label
|
| 8 |
Effect of genetic variations on ticagrelor plasma levels and clinical outcomes. Eur Heart J. 2015 Aug 1;36(29):1901-12.
|
| 9 |
Clinical pipeline report, company report or official report of AstraZeneca (2009).
|
| 10 |
KEGG: new perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017 Jan 4;45(D1):D353-D361. (dg:DG01665)
|
| 11 |
Absorption, distribution, metabolism, and excretion of ticagrelor in healthy subjects. Drug Metab Dispos. 2010 Sep;38(9):1514-21.
|
| 12 |
Genome-wide association study identifies ephrin type A receptors implicated in paclitaxel induced peripheral sensory neuropathy. J Med Genet. 2013 Sep;50(9):599-605. doi: 10.1136/jmedgenet-2012-101466. Epub 2013 Jun 17.
|
| 13 |
Pharmacoethnicity in Paclitaxel-Induced Sensory Peripheral Neuropathy. Clin Cancer Res. 2015 Oct 1;21(19):4337-46. doi: 10.1158/1078-0432.CCR-15-0133. Epub 2015 May 26.
|
| 14 |
Barbourin. A GPIIb-IIIa-specific integrin antagonist from the venom of Sistrurus m. barbouri. J Biol Chem. 1991 May 25;266(15):9359-62.
|
| 15 |
Eptifibatide in peripheral vascular interventions: results of the Integrilin Reduces Inflammation in Peripheral Vascular Interventions (INFLAME) trial. J Invasive Cardiol. 2006 Jan;18(1):6-12.
|
| 16 |
ADReCS-Target: target profiles for aiding drug safety research and application. Nucleic Acids Res. 2018 Jan 4;46(D1):D911-D917. doi: 10.1093/nar/gkx899.
|
|
|
|
|
|
|