Details of the Drug
General Information of Drug (ID: DMBR01X)
| Drug Name |
Brilinta
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Ticagrelor; 274693-27-5; Brilique; AZD-6140; AZD6140; AZD 6140; AR-C126532XX; UNII-GLH0314RVC; [14C]-Ticagrelor; GLH0314RVC; CHEBI:68558; (1S,2S,3R,5S)-3-[7-[[(1R,2S)-2-(3,4-DIFLUOROPHENYL)CYCLOPROPYL]AMINO]-5-(PROPYLTHIO)-3H-1,2,3-TRIAZOLO[4,5-D]PYRIMIDIN-3-YL]-5-(2-HYDROXYETHOXY)-1,2-CYCLOPENTANEDIOL; (1S,2S,3R,5S)-3-(7-(((1R,2S)-2-(3,4-difluorophenyl)cyclopropyl)amino)-5-(propylthio)-3H-[1,2,3]triazolo[4,5-d]pyrimidin-3-yl)-5-(2-hydroxyethoxy)cyclopentane-1,2-diol; Brilique; AR-C126532; Ticagrelor (USAN/INN); Brilinta/Brilique
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure |
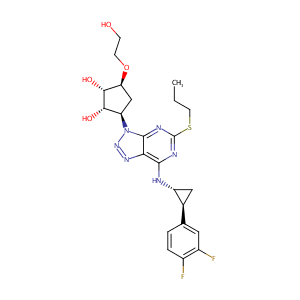 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 2 | Molecular Weight (mw) | 522.6 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 10 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 4 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 12 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Adverse Drug Reaction (ADR) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Transporter (DTP) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Brilinta (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Ticagrelor-Induced Syncope/Bradyarrhythmia. Cureus. 2021 Jan 23;13(1):e12874. | ||||
|---|---|---|---|---|---|
| 2 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 1765). | ||||
| 3 | ClinicalTrials.gov (NCT01732822) A Study Comparing Cardiovascular Effects of Ticagrelor and Clopidogrel in Patients With Peripheral Artery Disease. U.S. National Institutes of Health. | ||||
| 4 | Antithrombotic Therapy for Symptomatic Peripheral Arterial Disease: A Systematic Review and Network Meta-Analysis. Drugs. 2022 Aug;82(12):1287-1302. | ||||
| 5 | FDA Approved Drug Products: Brilinta Ticagrelor Oral Tablets | ||||
| 6 | BDDCS predictions, self-correcting aspects of BDDCS assignments, BDDCS assignment corrections, and classification for more than 175 additional drugs | ||||
| 7 | Absorption, distribution, metabolism, and excretion of ticagrelor in healthy subjects. Drug Metab Dispos. 2010 Sep;38(9):1514-21. doi: 10.1124/dmd.110.032250. Epub 2010 Jun 15. | ||||
| 8 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 9 | Effect of genetic variations on ticagrelor plasma levels and clinical outcomes. Eur Heart J. 2015 Aug 1;36(29):1901-12. | ||||
| 10 | Genome-wide association study identifies ephrin type A receptors implicated in paclitaxel induced peripheral sensory neuropathy. J Med Genet. 2013 Sep;50(9):599-605. doi: 10.1136/jmedgenet-2012-101466. Epub 2013 Jun 17. | ||||
| 11 | Pharmacoethnicity in Paclitaxel-Induced Sensory Peripheral Neuropathy. Clin Cancer Res. 2015 Oct 1;21(19):4337-46. doi: 10.1158/1078-0432.CCR-15-0133. Epub 2015 May 26. | ||||
| 12 | Clinical pipeline report, company report or official report of AstraZeneca (2009). | ||||
| 13 | KEGG: new perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017 Jan 4;45(D1):D353-D361. (dg:DG01665) | ||||
| 14 | Absorption, distribution, metabolism, and excretion of ticagrelor in healthy subjects. Drug Metab Dispos. 2010 Sep;38(9):1514-21. | ||||
| 15 | Canadian Pharmacists Association. | ||||
| 16 | Product Information. Brilinta (ticagrelor). Astra-Zeneca Pharmaceuticals, Wilmington, DE. | ||||
| 17 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 18 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 19 | Product Information. Balversa (erdafitinib). Janssen Products, LP, Horsham, PA. | ||||
| 20 | Product Information. Verzenio (abemaciclib). Lilly, Eli and Company, Indianapolis, IN. | ||||
| 21 | Product Information. Opsumit (macitentan). Actelion Pharmaceuticals US Inc, South San Francisco, CA. | ||||
| 22 | Product Information. Daurismo (glasdegib). Pfizer U.S. Pharmaceuticals Group, New York, NY. | ||||
| 23 | Alderman CP, Moritz CK, Ben-Tovim DI "Abnormal platelet aggregation associated with fluoxetine therapy." Ann Pharmacother 26 (1992): 1517-9. [PMID: 1482806] | ||||
| 24 | Product Information. Ofev (nintedanib). Boehringer Ingelheim, Ridgefield, CT. | ||||
| 25 | Patel S, Robinson R, Burk M "Hypertensive crisis associated with St. John's Wort." Am J Med 112 (2002): 507-8. [PMID: 11959071] | ||||
| 26 | Product Information. Polivy (polatuzumab vedotin). Genentech, South San Francisco, CA. | ||||
| 27 | Heck AM, DeWitt BA, Lukes AL "Potential interactions between alternative therapies and warfarin." Am J Health Syst Pharm 57 (2000): 1221-7 quiz 1228-30. [PMID: 10902065] | ||||
| 28 | Product Information. Aliqopa (copanlisib). Bayer Pharmaceutical Inc, West Haven, CT. | ||||
| 29 | Product Information. Tazverik (tazemetostat). Epizyme, Inc, Cambridge, MA. | ||||
| 30 | Product Information. Myrbetriq (mirabegron). Astellas Pharma US, Inc, Deerfield, IL. | ||||
| 31 | Product Information. Harvoni (ledipasvir-sofosbuvir). Gilead Sciences, Foster City, CA. | ||||
| 32 | Product Information. Pifeltro (doravirine). Merck & Company Inc, Whitehouse Station, NJ. | ||||
| 33 | Product Information. Tivicay (dolutegravir). ViiV Healthcare, Research Triangle Park, NC. | ||||
| 34 | Product Information. Juxtapid (lomitapide). Aegerion Pharmaceuticals Inc, Cambridge, MA. | ||||
| 35 | Product Information. Movantik (naloxegol). Astra-Zeneca Pharmaceuticals, Wilmington, DE. | ||||
| 36 | Product Information. Zepzelca (lurbinectedin). Jazz Pharmaceuticals, Palo Alto, CA. | ||||
| 37 | Product Information. Tagrisso (osimertinib). Astra-Zeneca Pharmaceuticals, Wilmington, DE. | ||||
| 38 | Product Information. Gavreto (pralsetinib). Blueprint Medicines Corporation, Cambridge, MA. | ||||
| 39 | Product Information. Tabrecta (capmatinib). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 40 | Caruso V, Iacoviello L, Di Castelnuovo A, et.al "Thrombotic complications in childhood acute lymphoblastic leukemia: a meta-analysis of 17 prospective studies comprising 1752 pediatric patients." Blood 108 (2006): 2216-22. [PMID: 16804111] | ||||
| 41 | Product Information. Calquence (acalabrutinib). Astra-Zeneca Pharmaceuticals, Wilmington, DE. | ||||
| 42 | Agencia Espaola de Medicamentos y Productos Sanitarios Healthcare "Centro de informacion online de medicamentos de la AEMPS - CIMA.". | ||||
| 43 | Product Information. Iclusig (ponatinib). Ariad Pharmaceuticals Inc, Cambridge, MA. | ||||
| 44 | Product Information. Braftovi (encorafenib). Array BioPharma Inc., Boulder, CO. | ||||
| 45 | Product Information. Ubrelvy (ubrogepant). Allergan Inc, Irvine, CA. | ||||
| 46 | Product Information. Reyvow (lasmiditan). Lilly, Eli and Company, Indianapolis, IN. | ||||
| 47 | Product Information. Varubi (rolapitant). Tesaro Inc., Waltham, MA. | ||||
| 48 | Product Information. Rozlytrek (entrectinib). Genentech, South San Francisco, CA. | ||||
| 49 | Product Information. Lynparza (olaparib). Astra-Zeneca Pharmaceuticals, Wilmington, DE. | ||||
| 50 | EMA. European Medicines Agency. European Union "EMA - List of medicines under additional monitoring.". | ||||
| 51 | Product Information. Xeglyze (abametapir topical). Dr. Reddy's Laboratories Inc, Upper Saddle River, NJ. | ||||
| 52 | Product Information. Xenleta (lefamulin). Nabriva Therapeutics US, Inc., King of Prussia, PA. | ||||
| 53 | Product Information. Orgovyx (relugolix). Myovant Sciences, Inc., Brisbane, CA. | ||||
| 54 | Product Information. Odomzo (sonidegib). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 55 | Abebe W "Herbal medication: potential for adverse interactions with analgesic drugs." J Clin Pharm Ther 27 (2002): 391-401. [PMID: 12472978] | ||||
| 56 | Product Information. Tavalisse (fostamatinib). Rigel Pharmaceuticals, South San Francisco, CA. | ||||
| 57 | Product Information. Cometriq (cabozantinib). Exelixis Inc, S San Francisco, CA. | ||||
| 58 | Product Information. Bevyxxa (betrixaban). Portola Pharmaceuticals, South San Francisco, CA. | ||||
