Details of the Drug
General Information of Drug (ID: DM03FHA)
| Drug Name |
LY293111
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Etalocib; LY293111; 161172-51-6; UNII-THY6RIW44R; LY 293111; THY6RIW44R; CHEMBL329123; LY-193111; 2-[3-[3-[2-ethyl-4-(4-fluorophenyl)-5-hydroxyphenoxy]propoxy]-2-propylphenoxy]benzoic acid; VML295; Etalocib [USAN:INN]; Etalocib (USAN); GTPL2948; SCHEMBL1649516; CTK8E7596; C33H33FO6; VML 295; DTXSID70167073; YFIZRWPXUYFCSN-UHFFFAOYSA-N; MolPort-009-019-411; ZINC3930629; AC1L4328; PDSP2_001221; BDBM50029450; PDSP1_001237; 1758AH; DB12850; RT-013626; D04074; L001468; J-009797; Benzoic acid, 2-(3-(3-((5-ethyl-4'-fluoro-2-hydroxy(1,1'-bipheny
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Anticancer Agents
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
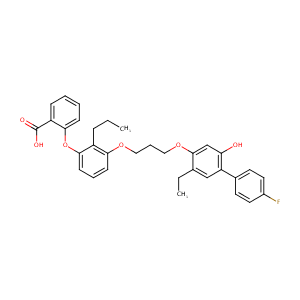 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 3 | Molecular Weight (mw) | 544.6 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 8.5 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 13 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 7 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Pancreatic cancer | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 2C10 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
