Details of the Drug
General Information of Drug (ID: DM0N19Y)
| Drug Name |
Chondroitin sulfate
|
|||||
|---|---|---|---|---|---|---|
| Synonyms |
Chondritinsulfate; Chondritinsulphate; Chondroitin 4 and 6 sulfate; Chondroitin 4-sulfate; Chondroitin 6-sulfate; Chondroitin polysulfate; Chondroitin sulfate; Chondroitin sulfuric acid; Chondroitin sulfuric acids; Chondroitin sulphate; Chondroitin, CHONDROITIN SULFATES; hydrogen sulfate; Chondroitine sulfate; Chondroitinsulfate C; Chondroitinsulfuric acids; Chondron; Chonsurid; Condrosan; Condrosulf; Sodium chondroitin sulfate; Structum; Uropol S; chondroitin sulfate C; 9007-28-7; EINECS 232-696-9; Gepan; UNII-6IC1M3OG5Z
|
|||||
| Affected Organisms |
Humans and other mammals
|
|||||
| ATC Code | ||||||
| Structure |
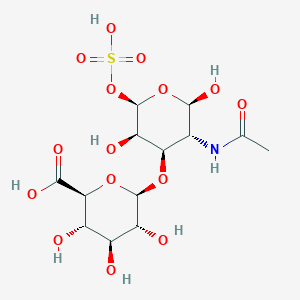 |
|||||
| 3D MOL | 2D MOL | |||||
| #Ro5 Violations (Lipinski): 3 | Molecular Weight (mw) | 463.37 | ||||
| Logarithm of the Partition Coefficient (xlogp) | -4.8 | |||||
| Rotatable Bond Count (rotbonds) | 6 | |||||
| Hydrogen Bond Donor Count (hbonddonor) | 8 | |||||
| Hydrogen Bond Acceptor Count (hbondacc) | 15 | |||||
| ADMET Property |
|
|||||
| Chemical Identifiers |
|
|||||
| Cross-matching ID | ||||||
Molecular Interaction Atlas of This Drug
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
