Details of the Drug
General Information of Drug (ID: DM28D05)
| Drug Name |
Oritavancin
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Nuvocid; Oritavancin [INN]; LY333328; Chlorobiphenyl-chloroeremomycin; (3S,6R,7R,22R,23S,26S,36R,38aR)-22-(3-Amino-2,3,6-trideoxy-3-C-methyl-alpha-L-mannopyranosyloxy)-3-(carbamoylmethyl)-10,19-dichloro-44-[2-O-[3-(4'-chlorobiphenyl-4-ylmethylamino)-2,3,6-trideoxy-3-C-me; (4''R)-22-O-(3-Amino-2,3,6-trideoxy-3-C-methyl-alpha-L-arabino-hexopyranosyl)-N3''-(4'-chloro[1,1'-biphenyl]-4-ylmethyl)vancomycin; (4''R)-22-O-(3-Amino-2,3,6-trideoxy-3-C-methyl-alpha-L-arabinohexopyranosyl)-N3''-(p-(p-chlorophenyl)benzyl)vancomycin
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Affected Organisms |
Gram-positive BacteriaStaphylococcus aureusEnterococcus faecalisStreptococcus pneumoniaeStreptococcus pyogenes
|
||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
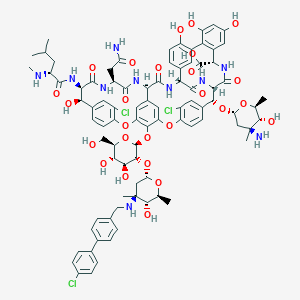 |
||||||||||||||||||||||||||
| 3D MOL is unavailable | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 4 | Molecular Weight (mw) | 1793.1 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 1.5 | ||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 19 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 20 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 29 | ||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Oritavancin (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
