Details of the Drug
General Information of Drug (ID: DM50ILT)
| Drug Name |
Fostemsavir
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
BMS-216; BMS-248; BMS-378806; BMS-626529; BMS-705; BMS-806; BMS-806 series; BMS-853; HIV-1 attachment inhibitors, BMS; HIV-1 entry inhibitors, BMS; BMS-488043 follow-on compounds, BMS; HIV-1 attachment inhibitors, Bristol-Myers Squibb
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Affected Organisms |
HIV-1
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
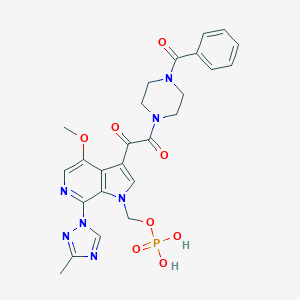 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 2 | Molecular Weight (mw) | 583.5 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -0.1 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 8 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 11 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Same Disease as Fostemsavir
Coadministration of a Drug Treating the Disease Different from Fostemsavir (Comorbidity)
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2020 | ||||
|---|---|---|---|---|---|
| 2 | Yu HZ, Han SF, Li P, Zhu CL, Zhang XX, Gan L, Gan Y: An examination of the potential effect of lipids on the first-pass metabolism of the lipophilic drug anethol trithione. J Pharm Sci. 2011 Nov;100(11):5048-58. doi: 10.1002/jps.22702. Epub 2011 Jul 15. | ||||
| 3 | FDA Approved Drug Products: Rukobia (fostemsavir) extended-release tablets | ||||
| 4 | Product Information. Rukobia (fostemsavir). ViiV Healthcare, Research Triangle Park, NC. | ||||
| 5 | Product Information. Fycompa (perampanel). Eisai Inc, Teaneck, NJ. | ||||
| 6 | Product Information. Xospata (gilteritinib). Astellas Pharma US, Inc, Deerfield, IL. | ||||
| 7 | Product Information. Arcapta Neohaler (indacaterol). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 8 | Product Information. Diabinese (chlorpropamide). Pfizer US Pharmaceuticals, New York, NY. | ||||
| 9 | Product Information. Daurismo (glasdegib). Pfizer U.S. Pharmaceuticals Group, New York, NY. | ||||
| 10 | Product Information. Nexletol (bempedoic acid). Esperion Therapeutics, Ann Arbor, MI. | ||||
| 11 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 12 | Product Information. Retevmo (selpercatinib). Lilly, Eli and Company, Indianapolis, IN. | ||||
| 13 | Product Information. Nurtec ODT (rimegepant). Biohaven Pharmaceuticals, New Haven, CT. | ||||
| 14 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 15 | Product Information. Zeposia (ozanimod). Celgene Corporation, Summit, NJ. | ||||
| 16 | EMA. European Medicines Agency. European Union "EMA - List of medicines under additional monitoring.". | ||||
