Details of the Drug
General Information of Drug (ID: DMIJC9X)
| Drug Name |
Osilodrostat
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
LCI699; 928134-65-0; LCI-699; UNII-5YL4IQ1078; CHEMBL3099695; 5YL4IQ1078; Osilodrostat;4-[(5R)-6,7-dihydro-5H-pyrrolo[1,2-c]imidazol-5-yl]-3-fluorobenzonitrile; 1304733-26-3; Osilodrostat [USAN:INN]; LCI 699; Osilodrostat (USAN/INN); LCI699-NX; GTPL8310; SCHEMBL12460772; DTXSID40156570; MolPort-044-560-410; EX-A1397; ZINC72318114; s7456; BDBM50444549; AKOS027323750; DB11837; CS-6896; HY-16276; KB-78108; Z-3271; D11061; 4-[(5R)-6,7-Dihydro-5H-pyrrolo[1,2-c]imidazol-5-yl]-3-fluorobenzo nitrile
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
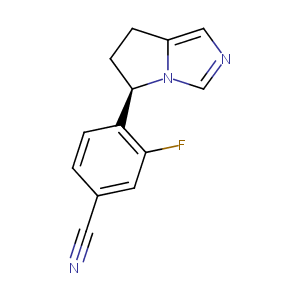 |
||||||||||||||||||||||
| 3D MOL is unavailable | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 227.24 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 1.9 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Cushing disease | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 5A70 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Osilodrostat (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health Human Services. 2020 | ||||
|---|---|---|---|---|---|
| 2 | FDA Approved Drug Products: Isturisa (osilodrostat) oral tablets | ||||
| 3 | EMA Summary of Product Characteristics: Isturisa (osilodrostat) oral tablets | ||||
| 4 | Product Information. Fycompa (perampanel). Eisai Inc, Teaneck, NJ. | ||||
| 5 | Product Information. Xospata (gilteritinib). Astellas Pharma US, Inc, Deerfield, IL. | ||||
| 6 | Product Information. Arcapta Neohaler (indacaterol). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 7 | Product Information. Isturisa (osilodrostat). Recordati Rare Diseases Inc, Lebanon, NJ. | ||||
| 8 | Product Information. Verzenio (abemaciclib). Lilly, Eli and Company, Indianapolis, IN. | ||||
| 9 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 10 | Product Information. Austedo (deutetrabenazine). Teva Pharmaceuticals USA, North Wales, PA. | ||||
| 11 | Product Information. Diabinese (chlorpropamide). Pfizer US Pharmaceuticals, New York, NY. | ||||
| 12 | Product Information. Pifeltro (doravirine). Merck & Company Inc, Whitehouse Station, NJ. | ||||
| 13 | Product Information. Orladeyo (berotralstat). BioCryst Pharmaceuticals Inc, Durham, NC. | ||||
| 14 | Product Information. Accolate (zafirlukast). Zeneca Pharmaceuticals, Wilmington, DE. | ||||
| 15 | Product Information. Zepzelca (lurbinectedin). Jazz Pharmaceuticals, Palo Alto, CA. | ||||
| 16 | Product Information. Gavreto (pralsetinib). Blueprint Medicines Corporation, Cambridge, MA. | ||||
| 17 | Product Information. Retevmo (selpercatinib). Lilly, Eli and Company, Indianapolis, IN. | ||||
| 18 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 19 | Product Information. Zeposia (ozanimod). Celgene Corporation, Summit, NJ. | ||||
| 20 | Product Information. Xeglyze (abametapir topical). Dr. Reddy's Laboratories Inc, Upper Saddle River, NJ. | ||||
| 21 | Product Information. Macrilen (macimorelin). Aeterna Zentaris, Charleston, SC. | ||||
| 22 | Product Information. Nubeqa (darolutamide). Bayer HealthCare Pharmaceuticals Inc., Whippany, NJ. | ||||
