Details of the Drug
General Information of Drug (ID: DM7U5QJ)
| Drug Name |
Cinnarizine
|
||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Aplactan; Aplexal; Apotomin; Artate; Carecin; Cerebolan; Cinaperazine; Cinarizina; Cinarizine; Cinazyn; Cinnacet; Cinnageron; Cinnarizinum; Corathiem; Denapol; Dimitron; Dimitronal; Eglen; Folcodal; Giganten; Glanil; Hilactan; Ixertol; Katoseran; Labyrin; Lazeta; Libotasin; Marisan; Midronal; Mitronal; Olamin; Processine; Sedatromin; Senoger; Sepan; Siptazin; Spaderizine; Stugeron; Stutgeron; Stutgin; Toliman; Votegol; Zepamol; Cinnarizine Stugeron; C 5270; MD 516; R 1575; R 516; Cero-Aterin; Cinarizina [INN-Spanish]; Cinnarizinum [INN-Latin]; R-516; Stugeron (TN); Stunarone (TN); Cinnarizine (JAN/USAN/INN); Cinnarizine [USAN:INN:BAN:JAN]; N-Benzhydryl-N'-cinnamylpiperazine; 1-(Diphenylmethyl)-4-(3-phenyl-2-propenyl)piperazine; 1-(diphenylmethyl)-4-(3-phenyl-2-propenyl)-piperazine; 1-(diphenylmethyl)-4-[(2E)-3-phenylprop-2-en-1-yl]piperazine; 1-Benzhydryl-4-cinnamylpiperazin; 1-Cinnamyl-4-(diphenylmethyl)piperazine; 1-benzhydryl-4-[(E)-3-phenylprop-2-enyl]piperazine; 1-trans-Cinnamyl-4-diphenylmethylpiperazine
|
||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||
| Therapeutic Class |
Antiallergic Agents
|
||||||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||
| Structure |
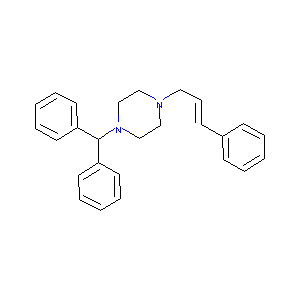 |
||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 368.5 | |||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 5.8 | ||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 6 | ||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 2 | ||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Meniere disease | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | AB31.0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
