Details of the Drug
General Information of Drug (ID: DM8SMD1)
| Drug Name |
Tazarotene
|
||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Avage; Suretin; Tazaroteno; Tazarotenum; Tazorac; Tazoral; Zorac; AGN 190168; AGN-190168; Avage (TN); Tazarotene [USAN:INN]; Tazorac (TN); Zorac (TN); Tazarotene (JAN/USAN/INN); Tazorac, Avage, Zora, Tazarotene; Ethyl 6-((4,4-dimethylthiochroman-6-yl)ethynyl)nicotinate; Ethyl 6-(2-(4,4-dimethylthiochroman-6-yl)ethynyl)nicotinate; Ethyl 6-[(4,4-dimethyl-3,4-dihydro-2H-thiochromen-6-yl)ethynyl]nicotinate; Ethyl 6-[2-(4,4-dimethyl-2,3-dihydrothiochromen-6-yl)ethynyl]pyridine-3-carboxylate; 3-Pyridinecarboxylic acid, 6-((3,4-dihydro-4,4-dimethyl-2H-1-benzothiopyran-6-yl)ethynyl)-, ethyl ester; 3-Pyridinecarboxylic acid, 6-((3,4-dihydro-4,4-dimethyl-2H-1-benzothiopyran-6-yl)ethynyl)-,ethyl ester
|
||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||
| Therapeutic Class |
Dermatologic Agents
|
||||||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||
| Structure |
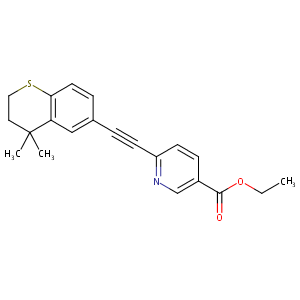 |
||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 351.5 | |||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 4.9 | ||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 5 | ||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 4 | ||||||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| ICD Disease Classification | 14 Disease of the skin | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Disease Class | ICD-11: EA90 Psoriasis | |||||||||||||||||||||||||||||
| The Studied Tissue | Skin | |||||||||||||||||||||||||||||
| The Studied Disease | Psoriasis [ICD-11:EA90] | |||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||
References
