Details of the Drug
General Information of Drug (ID: DMC3YUA)
| Drug Name |
Benzbromarone
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Acifugan; Azubromaron; Benzbromaron; Benzbromaronratiopharm; Benzbromaronum; Benzobromarona; Besuric; Desuric; Exurate; Harolan; Hipurik; Minuric; Narcaricin; Normurat; Uricovac; Urinorm; Uroleap; Aliud Brand of Benzbromarone; Benzbromaron AL; Benzbromaron ratiopharm; Benzbromarone Aliud Brand; Benzbromarone Heumann Brand; Benzbromarone Sanfer Brand; Benzbromarone ratiopharm Brand; Heumann Brand of Benzbromarone; Ratiopharm Brand of Benzbromarone; Sanfer Brand of Benzbromarone; Sanofi Winthrop Brand of Benzbromarone; L 2214; L2214; MJ 10061; NCI85433; AL, Benzbromaron; Benzbromaron-ratiopharm; Benzbromaronum [INN-Latin]; Benzobromarona [INN-Spanish]; L 2214-Labaz; L-2214; Uroleap (TN); Benzbromarone [USAN:INN:BAN]; Methanone, (3; Benzbromarone (JP15/USAN/INN); KETONE, 3,5-DIBROMO-4-HYDROXYPHENYL 2-ETHYL-3-BENZOFURANYL; Ketone, 3,5-dibromo-4-hydroxyphenyl 2-ethyl-3-benzofuranyl; (2-Ethyl-3-benzofuranyl)-(3,5-dibrom-4-hydroxyphenyl)keton; (3,5-Dibromo-4-hydroxyphenyl)(2-ethyl-1-benzofuran-3-yl)methanone; (3,5-dibromo-4-hydroxyphenyl)-(2-ethyl-1-benzofuran-3-yl)methanone; 2-Ethyl-3-(3,5-dibrom-4-hydroxybenzoyl)benzofuran; 3, 5-Dibromo-4-hydroxyphenyl-2-ethyl-3-benzofuranyl ketone; 3,5-Dibromo-4-hydroxyphenyl-2-ethyl-3-benzofuranyl ketone; 3-(3,5-Dibromo-4-hydroxybenzoyl)-2-ethylbenzofuran; 3-[3,5-DIBROMO-4-HYDROXYBENZOYL]-2-ETHYLBENZOFURAN
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
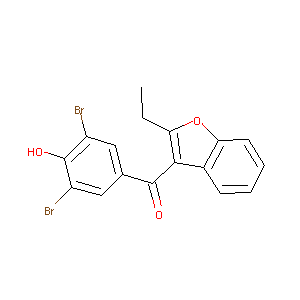 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 424.1 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 5.7 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Transporter (DTP) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Gout | |||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | FA25 | |||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||||||||
References
