Details of the Drug
General Information of Drug (ID: DMDO279)
| Drug Name |
Phenprocoumon
|
||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Falithiom; Falithrom; Fencumar; Fenprocoumona; Fenprocumon; Fenprocumone; Liquamar; Marcoumar; Marcumar; Marcuphen; Phenprocoumalol; Phenprocoumarol; Phenprocoumarole; Phenprocoumone; Phenprocoumonum; Phenprocumone; Phenprocumonum; Phenprogramma; Phenylpropylhydroxycumarinum; Fenprocumone [DCIT]; Hexal Brand of Phenprocoumon; Roche Brand of Phenprocoumon; Worwag Brand of Phenprocoumon; U29342; Falithrom (TN); Fenprocoumona [INN-Spanish]; Liquamar (TN); Marcoumar (TN); Marcumar (TN); Phenprocoumone [INN-French]; Phenprocoumonum [INN-Latin]; Ro 1-4849; Phenprocoumon (USAN/INN); Phenprocoumon [USAN:INN:BAN]; 2-hydroxy-3-(1-phenylpropyl)chromen-4-one; 3-(1'-Phenyl-propyl)-4-oxycoumarin; 3-(1'-Phenyl-propyl)-4-oxycoumarin [German]; 3-(1-Phenylpropyl)-4-hydroxycoumarin; 3-(alpha-Ethylbenzyl)-4-hydroxycoumarin; 3-(alpha-Phenylpropyl)-4-hydroxycoumarin; 4-Hydroxy-3-(1-phenylpropyl)-2H-1-benzopyran-2-one; 4-Hydroxy-3-(1-phenylpropyl)-2H-chromen-2-one
|
||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||
| Therapeutic Class |
Anticoagulants
|
||||||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||
| Structure |
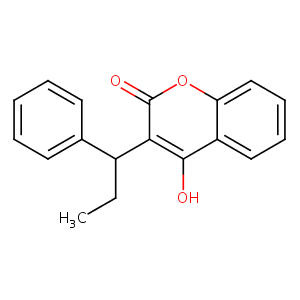 |
||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 280.3 | |||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3.6 | ||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 3 | ||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | ||||||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Transporter (DTP) |
|
||||||||||||||||||||||||||||||||||||
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||
References
