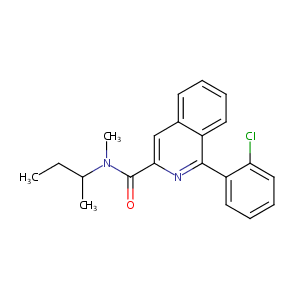
|
Cancer Drug Sensitivity Data Curated from 1 Cell Line(s) in Bone Tissue
| Cell Line |
Original Dataset |
IC50 |
IC90 |
EC50 |
AUC |
|
U2OS
|
CTRP1
|
10.6069
|
14.3664
|
7.5576
|
8.1404
|
| ----------- |
|
|
|
|
|
Cancer Drug Sensitivity Data Curated from 1 Cell Line(s) in Breast Tissue
| Cell Line |
Original Dataset |
IC50 |
IC90 |
EC50 |
AUC |
|
MCF-7
|
CTRP1
|
6.4789
|
9.095
|
6.2333
|
38.2912
|
| ----------- |
|
|
|
|
|
Cancer Drug Sensitivity Data Curated from 2 Cell Line(s) in Central Nervous System Tissue
| Cell Line |
Original Dataset |
IC50 |
IC90 |
EC50 |
AUC |
|
LN-229
|
CTRP1
|
6.7221
|
8.6162
|
6.6145
|
34.1207
|
|
GaMG
|
CTRP1
|
7.2642
|
13.8646
|
2.3306
|
14.8631
|
| ----------- |
|
|
|
|
|
Cancer Drug Sensitivity Data Curated from 6 Cell Line(s) in Endometrium Tissue
| Cell Line |
Original Dataset |
IC50 |
IC90 |
EC50 |
AUC |
|
HEC-151
|
CTRP1
|
5.6048
|
7.3065
|
5.5871
|
49.3579
|
|
KLE
|
CTRP1
|
6.3815
|
10.9888
|
3.1107
|
12.2202
|
|
JHUEM-2
|
CTRP1
|
6.6124
|
8.0862
|
6.5783
|
35.1856
|
|
HEC-59
|
CTRP1
|
7.251
|
8.5474
|
4.6427
|
0.2028
|
|
HEC-108
|
CTRP1
|
9.6071
|
17.9387
|
1.8983
|
11.7508
|
|
Ishikawa (Heraklio) 02 ER-
|
CTRP1
|
6.0297
|
10.4181
|
3.139
|
13.1535
|
| ----------- |
|
|
|
|
|
| ⏷ Show the Full List of 6 Cancer Drug Sensitivity Data of This Drug |
|
Cancer Drug Sensitivity Data Curated from 19 Cell Line(s) in Haematopoietic And Lymphoid Tissue Tissue
| Cell Line |
Original Dataset |
IC50 |
IC90 |
EC50 |
AUC |
|
LP-1
|
CTRP1
|
5.0816
|
7.7933
|
4.987
|
56.0465
|
|
GDM-1
|
CTRP1
|
5.3686
|
7.3278
|
5.3406
|
52.6128
|
|
PL21
|
CTRP1
|
5.3743
|
7.8152
|
5.2965
|
52.4199
|
|
NOMO-1
|
CTRP1
|
5.6533
|
7.3103
|
5.637
|
48.6771
|
|
BL-70
|
CTRP1
|
5.7015
|
6.8523
|
4.6242
|
1.9236
|
|
U-937
|
CTRP1
|
5.7372
|
10.7898
|
4.8486
|
48.3661
|
|
OCI-AML-3
|
CTRP1
|
5.7425
|
8.4191
|
5.5921
|
47.6586
|
|
KMS-11
|
CTRP1
|
5.7496
|
7.8619
|
5.69
|
47.4151
|
|
NU-DUL-1
|
CTRP1
|
5.9714
|
7.776
|
5.934
|
44.2774
|
|
SKM-1
|
CTRP1
|
6.1061
|
7.5501
|
6.0915
|
42.2676
|
|
RPMI-8226
|
CTRP1
|
6.3398
|
7.7354
|
6.3225
|
38.9581
|
|
MOLM-16
|
CTRP1
|
6.3713
|
8.1602
|
6.3134
|
38.7575
|
|
THP-1
|
CTRP1
|
6.3982
|
8.0362
|
6.3573
|
38.2746
|
|
WSU-DLCL2
|
CTRP1
|
6.6206
|
7.6389
|
6.6158
|
34.8672
|
|
SU-DHL-5
|
CTRP1
|
6.6384
|
7.2565
|
6.6383
|
34.5785
|
|
EJM
|
CTRP1
|
6.7535
|
8.4897
|
6.6724
|
33.499
|
|
SU-DHL-4
|
CTRP1
|
7.2081
|
8.1202
|
7.1986
|
26.5077
|
|
AMO1
|
CTRP1
|
7.8143
|
8.5126
|
7.8019
|
17.8688
|
|
OCI-Ly10
|
CTRP1
|
7.8955
|
11.0083
|
7.0013
|
23.6727
|
| ----------- |
|
|
|
|
|
| ⏷ Show the Full List of 19 Cancer Drug Sensitivity Data of This Drug |
|
Cancer Drug Sensitivity Data Curated from 1 Cell Line(s) in Kidney Tissue
| Cell Line |
Original Dataset |
IC50 |
IC90 |
EC50 |
AUC |
|
RCC10RGB
|
CTRP1
|
6.5336
|
7.856
|
6.5157
|
36.1997
|
| ----------- |
|
|
|
|
|
Cancer Drug Sensitivity Data Curated from 27 Cell Line(s) in Large Intestine Tissue
| Cell Line |
Original Dataset |
IC50 |
IC90 |
EC50 |
AUC |
|
CW-2
|
CTRP1
|
5.7403
|
8.46
|
5.5814
|
47.699
|
|
SW480
|
CTRP1
|
5.8309
|
8.2437
|
5.7205
|
46.4246
|
|
SNU-C5
|
CTRP1
|
6.3673
|
8.4985
|
6.2533
|
39.1276
|
|
SNU-C4
|
CTRP1
|
6.3828
|
8.0858
|
6.3353
|
38.5327
|
|
SNU-175
|
CTRP1
|
6.5193
|
8.4485
|
6.427
|
36.8785
|
|
SNU-81
|
CTRP1
|
6.6025
|
8.3173
|
6.5381
|
35.528
|
|
SNU-61
|
CTRP1
|
6.6183
|
8.2795
|
6.5607
|
35.2589
|
|
SW620
|
CTRP1
|
6.7548
|
8.6382
|
6.6454
|
33.671
|
|
HT115
|
CTRP1
|
6.8098
|
8.3766
|
6.7514
|
32.5419
|
|
HCT 116
|
CTRP1
|
6.8474
|
8.3063
|
6.8015
|
31.9192
|
|
NCI-H508
|
CTRP1
|
6.9664
|
8.2707
|
6.9324
|
30.1364
|
|
LS180
|
CTRP1
|
7.0139
|
8.4063
|
6.9655
|
29.5627
|
|
SNU-1040
|
CTRP1
|
7.0177
|
8.2599
|
6.988
|
29.3736
|
|
HT-55
|
CTRP1
|
7.0413
|
8.3202
|
7.006
|
29.0772
|
|
RKO
|
CTRP1
|
7.0785
|
8.1656
|
7.0607
|
28.4196
|
|
RCM-1 [Human ESC]
|
CTRP1
|
7.1079
|
8.9462
|
6.9594
|
28.9504
|
|
HCT 8
|
CTRP1
|
7.124
|
8.1703
|
7.1079
|
27.7573
|
|
COLO 678
|
CTRP1
|
7.1294
|
8.5925
|
7.0597
|
28.0702
|
|
COLO 320
|
CTRP1
|
7.146
|
11.5411
|
6.0054
|
34.4572
|
|
LoVo
|
CTRP1
|
7.1821
|
8.6272
|
7.1103
|
27.3341
|
|
SW948
|
CTRP1
|
7.2668
|
9.0308
|
7.1108
|
26.7523
|
|
MDST8
|
CTRP1
|
7.2725
|
8.1177
|
7.2652
|
25.5725
|
|
KM12
|
CTRP1
|
7.3268
|
8.6311
|
7.2659
|
25.1896
|
|
CL-34
|
CTRP1
|
7.6536
|
8.4823
|
7.6359
|
20.2038
|
|
HT-29
|
CTRP1
|
7.8424
|
8.5405
|
7.8289
|
17.4761
|
|
LS123
|
CTRP1
|
7.882
|
9.2182
|
7.7277
|
17.9842
|
|
CL-40
|
CTRP1
|
8.0187
|
9.117
|
7.9073
|
15.6994
|
| ----------- |
|
|
|
|
|
| ⏷ Show the Full List of 27 Cancer Drug Sensitivity Data of This Drug |
|
Cancer Drug Sensitivity Data Curated from 4 Cell Line(s) in Liver Tissue
| Cell Line |
Original Dataset |
IC50 |
IC90 |
EC50 |
AUC |
|
JHH-6
|
CTRP1
|
6.0502
|
9.5179
|
3.5917
|
9.4984
|
|
SNU-449
|
CTRP1
|
6.1561
|
8.069
|
4.34
|
3.1037
|
|
SNU-398
|
CTRP1
|
6.5175
|
8.0068
|
6.4863
|
36.5173
|
|
Hep-G2
|
CTRP1
|
7.7486
|
8.393
|
7.742
|
18.766
|
| ----------- |
|
|
|
|
|
Cancer Drug Sensitivity Data Curated from 44 Cell Line(s) in Lung Tissue
| Cell Line |
Original Dataset |
IC50 |
IC90 |
EC50 |
AUC |
|
NCI-H1963
|
CTRP1
|
5.3515
|
7.3871
|
5.3182
|
52.8292
|
|
NCI-H1435
|
CTRP1
|
5.6816
|
7.6658
|
4.2308
|
5.2426
|
|
SCLC-21H
|
CTRP1
|
6.0955
|
6.963
|
6.095
|
42.3375
|
|
HCC1833
|
CTRP1
|
6.0992
|
8.3199
|
5.9965
|
42.7704
|
|
NCI-H2342
|
CTRP1
|
6.1314
|
7.9376
|
6.086
|
42.0676
|
|
NCI-H2286
|
CTRP1
|
6.3515
|
8.8531
|
4.1001
|
4.5201
|
|
COLO 699
|
CTRP1
|
6.3531
|
7.9914
|
6.3145
|
38.8994
|
|
HCC2108
|
CTRP1
|
6.4268
|
7.4621
|
6.4233
|
37.6261
|
|
NCI-H441
|
CTRP1
|
6.5015
|
8.3228
|
6.429
|
37.0079
|
|
T3M-10
|
CTRP1
|
6.5053
|
8.2175
|
6.4486
|
36.8553
|
|
NCI-H1836
|
CTRP1
|
6.5067
|
8.1545
|
6.4584
|
36.7816
|
|
HCC1359
|
CTRP1
|
6.5238
|
7.1265
|
6.5237
|
36.2165
|
|
NCI-H596
|
CTRP1
|
6.593
|
8.126
|
6.5534
|
35.4987
|
|
NCI-H2122
|
CTRP1
|
6.6883
|
8.0889
|
6.6581
|
34.0773
|
|
LXF 289
|
CTRP1
|
6.7627
|
12.3317
|
2.7258
|
13.785
|
|
NCI-H1915
|
CTRP1
|
6.7741
|
10.9122
|
3.4084
|
8.8326
|
|
RERF-LC-KJ
|
CTRP1
|
6.8599
|
12.2478
|
2.832
|
12.7724
|
|
NCI-H2141
|
CTRP1
|
6.9011
|
7.6746
|
6.8995
|
30.837
|
|
CAL-12T
|
CTRP1
|
6.9084
|
8.4263
|
6.8496
|
31.1414
|
|
NCI-H841
|
CTRP1
|
6.9268
|
8.2446
|
6.8935
|
30.6967
|
|
NCI-H2081
|
CTRP1
|
6.9507
|
8.0933
|
6.9329
|
30.2449
|
|
COLO 668
|
CTRP1
|
6.9515
|
10.5044
|
3.7049
|
6.1467
|
|
NCI-H2073
|
CTRP1
|
6.9857
|
9.2526
|
6.7407
|
31.347
|
|
HCC15
|
CTRP1
|
7.0242
|
8.5117
|
6.9603
|
29.5275
|
|
HCC4006
|
CTRP1
|
7.0718
|
8.1653
|
7.0538
|
28.5165
|
|
NCI-H2172
|
CTRP1
|
7.158
|
9.1252
|
6.9659
|
28.5583
|
|
NCI-H1105
|
CTRP1
|
7.1683
|
8.0555
|
7.1609
|
27.0619
|
|
NCI-H1755
|
CTRP1
|
7.1787
|
8.4776
|
7.1314
|
27.203
|
|
HCC2935
|
CTRP1
|
7.2097
|
8.663
|
7.1335
|
26.9732
|
|
NCI-H1930
|
CTRP1
|
7.2099
|
9.0155
|
4.4598
|
0.8266
|
|
HCC1195
|
CTRP1
|
7.2421
|
9.1176
|
7.0598
|
27.2976
|
|
NCI-H1876
|
CTRP1
|
7.2814
|
8.3208
|
7.2598
|
25.5492
|
|
NCI-H2023
|
CTRP1
|
7.2823
|
8.755
|
7.1937
|
26.0295
|
|
NCI-H1694
|
CTRP1
|
7.2826
|
8.4964
|
7.24
|
25.6852
|
|
NCI-H1781
|
CTRP1
|
7.3495
|
8.7149
|
7.2748
|
24.9672
|
|
NCI-H460
|
CTRP1
|
7.3767
|
7.8628
|
7.3764
|
24.0333
|
|
SK-LU-1
|
CTRP1
|
7.3983
|
8.3201
|
7.3825
|
23.8367
|
|
COR-L51
|
CTRP1
|
7.4073
|
8.3187
|
7.3921
|
23.7039
|
|
NCI-H2030
|
CTRP1
|
7.4391
|
8.6833
|
7.3778
|
23.5889
|
|
NCI-H3255
|
CTRP1
|
7.4747
|
9.7966
|
7.0845
|
25.5843
|
|
HCC827
|
CTRP1
|
7.7848
|
8.7362
|
7.7414
|
18.5191
|
|
Sq-1
|
CTRP1
|
8.0294
|
8.9576
|
7.9613
|
15.2135
|
|
COR-L23
|
CTRP1
|
8.1787
|
8.9455
|
8.1267
|
12.9598
|
|
NCI-H2110
|
CTRP1
|
9.5499
|
15.2659
|
6.3545
|
20.387
|
| ----------- |
|
|
|
|
|
| ⏷ Show the Full List of 44 Cancer Drug Sensitivity Data of This Drug |
|
Cancer Drug Sensitivity Data Curated from 23 Cell Line(s) in Ovary Tissue
| Cell Line |
Original Dataset |
IC50 |
IC90 |
EC50 |
AUC |
|
COV434
|
CTRP1
|
4.9589
|
6.9162
|
4.9412
|
58.2169
|
|
OVCAR-8
|
CTRP1
|
5.6903
|
7.8625
|
5.6269
|
48.2288
|
|
OVCAR-4
|
CTRP1
|
5.7667
|
8.4551
|
5.611
|
47.3541
|
|
OVK18
|
CTRP1
|
5.985
|
7.74
|
5.9517
|
44.0696
|
|
RMUG-S
|
CTRP1
|
6.0367
|
8.7168
|
5.8474
|
43.9225
|
|
COV318
|
CTRP1
|
6.4689
|
13.2514
|
2.0701
|
18.8327
|
|
A2780
|
CTRP1
|
6.5923
|
8.68
|
6.4602
|
36.0986
|
|
OV-90
|
CTRP1
|
6.6432
|
8.3841
|
6.5715
|
34.9989
|
|
JHOM-1
|
CTRP1
|
6.7014
|
8.2356
|
6.6552
|
34.0012
|
|
OVMANA
|
CTRP1
|
7.0238
|
8.5128
|
4.5708
|
0.5186
|
|
RMG-I
|
CTRP1
|
7.1039
|
9.0597
|
6.9251
|
29.2259
|
|
OVKATE
|
CTRP1
|
7.1248
|
8.3448
|
7.0917
|
27.8692
|
|
TYK-nu
|
CTRP1
|
7.1434
|
8.1247
|
7.1313
|
27.4511
|
|
SNU-840
|
CTRP1
|
7.2373
|
8.4947
|
7.1917
|
26.3543
|
|
COV644
|
CTRP1
|
7.2746
|
8.2922
|
7.2554
|
25.6283
|
|
IGROV-1
|
CTRP1
|
7.2832
|
8.6317
|
7.2187
|
25.8376
|
|
JHOC-5
|
CTRP1
|
7.3816
|
8.5918
|
7.3315
|
24.3267
|
|
Caov-3
|
CTRP1
|
7.4372
|
8.0076
|
7.4362
|
23.1746
|
|
OAW42
|
CTRP1
|
7.5602
|
7.9835
|
7.56
|
21.4111
|
|
OV7
|
CTRP1
|
7.8058
|
8.5568
|
7.7888
|
18.0244
|
|
JHOS-4
|
CTRP1
|
7.9125
|
15.6543
|
1.9089
|
15.5434
|
|
EFO-27
|
CTRP1
|
8.2647
|
11.7092
|
7.025
|
21.4837
|
|
COV362
|
CTRP1
|
8.4578
|
9.1188
|
8.3856
|
9.1338
|
| ----------- |
|
|
|
|
|
| ⏷ Show the Full List of 23 Cancer Drug Sensitivity Data of This Drug |
|
Cancer Drug Sensitivity Data Curated from 1 Cell Line(s) in Pancreas Tissue
| Cell Line |
Original Dataset |
IC50 |
IC90 |
EC50 |
AUC |
|
PaTu 8902
|
CTRP1
|
7.1667
|
9.2982
|
4.3327
|
1.4932
|
| ----------- |
|
|
|
|
|
Cancer Drug Sensitivity Data Curated from 1 Cell Line(s) in Prostate Tissue
| Cell Line |
Original Dataset |
IC50 |
IC90 |
EC50 |
AUC |
|
PaCa-3
|
CTRP1
|
7.3304
|
16.7671
|
0.9729
|
21.7185
|
| ----------- |
|
|
|
|
|
Cancer Drug Sensitivity Data Curated from 5 Cell Line(s) in Skin Tissue
| Cell Line |
Original Dataset |
IC50 |
IC90 |
EC50 |
AUC |
|
SK-MEL-5
|
CTRP1
|
7.1926
|
7.9338
|
7.19
|
26.6798
|
|
A-375
|
CTRP1
|
7.3213
|
8.174
|
7.3126
|
24.8855
|
|
SK-MEL-1
|
CTRP1
|
7.8193
|
9.4351
|
7.5874
|
19.4905
|
|
SK-MEL-28
|
CTRP1
|
7.8782
|
8.3547
|
7.8763
|
16.8805
|
|
Hs 852.T
|
CTRP1
|
9.1271
|
20.937
|
0.2007
|
20.3178
|
| ----------- |
|
|
|
|
|
Cancer Drug Sensitivity Data Curated from 2 Cell Line(s) in Soft Tissue Tissue
| Cell Line |
Original Dataset |
IC50 |
IC90 |
EC50 |
AUC |
|
HT-1080
|
CTRP1
|
7.3563
|
8.3806
|
7.3327
|
24.4931
|
|
RKN
|
CTRP1
|
7.4639
|
9.1827
|
7.283
|
24.1413
|
| ----------- |
|
|
|
|
|
Cancer Drug Sensitivity Data Curated from 3 Cell Line(s) in Stomach Tissue
| Cell Line |
Original Dataset |
IC50 |
IC90 |
EC50 |
AUC |
|
AGS
|
CTRP1
|
6.6579
|
8.0404
|
6.6308
|
34.4898
|
|
MKN74
|
CTRP1
|
7.0384
|
9.4042
|
4.2302
|
2.2666
|
|
SNG-M
|
CTRP1
|
6.3312
|
8.7735
|
4.1242
|
4.3791
|
| ----------- |
|
|
|
|
|
Cancer Drug Sensitivity Data Curated from 1 Cell Line(s) in Upper Aerodigestive Tract Tissue
| Cell Line |
Original Dataset |
IC50 |
IC90 |
EC50 |
AUC |
|
SCC-9
|
CTRP1
|
7.5075
|
8.5558
|
7.4719
|
22.4218
|
| ----------- |
|
|
|
|
|
|