Details of the Drug
General Information of Drug (ID: DMENSL5)
| Drug Name |
Aminosalicylic Acid
|
||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
4-Aminosalicylic acid; 65-49-6; Aminosalicylic acid; P-AMINOSALICYLIC ACID; Rezipas; Aminopar; Pamisyl; Parasalindon; Ferrosan; Deapasil; Apacil; Parasal; Paser; Paramycin; Gabbropas; Parasalicil; Pasnodia; Osacyl; Aminox; Pasolac; Pasmed; Propasa; Pasalon; Entepas; Pasdium; Pasara; Pamacyl; Pasem; Pasa; 2-Hydroxy-4-aminobenzoic acid; PASK; APAS; Para-Pas; Sanipirol-4; Hellipidyl; Pascorbic; PAS-C; Benzoic acid, 4-amino-2-hydroxy-; PAS (acid); Aminosalicylic Acid Resin Complex; Aminosalicylate Sodium
|
||||||||||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||||||||||
| Affected Organisms |
Mycobacteria
|
||||||||||||||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||||||||||
| Structure |
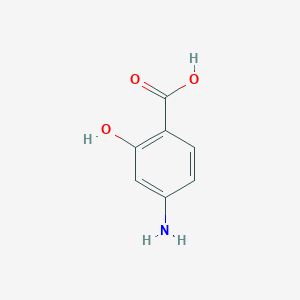 |
||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 153.14 | |||||||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 1.3 | ||||||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 1 | ||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 3 | ||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 4 | ||||||||||||||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Transporter (DTP) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Crohn disease | |||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | DD70 | |||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Aminosalicylic Acid (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
