| Drug Name |
MURAGLITAZAR
|
| Synonyms |
Muraglitazar; 331741-94-7; Pargluva; BMS-298585; UNII-W1MKM70WQI; BMS 298585; W1MKM70WQI; CHEMBL186179; N-[(4-Methoxyphenoxy)carbonyl]-N-[[4-[2-(5-methyl-2-phenyl-4-oxazolyl)ethoxy]phenyl]methyl]glycine; N-((4-methoxyphenoxy)carbonyl)-N-((4-(2-(5-methyl-2-phenyl-4-oxazolyl)ethoxy)phenyl)methyl)glycine; BMS298585; Muraglitazar [USAN:INN]; CCRIS 9258; AC1L4FVP; Muraglitazar (USAN/INN); DSSTox_CID_31508; DSSTox_RID_97393; DSSTox_GSID_57719; SCHEMBL676469; DTXSID9057719; CTK8E8901; MolPort-006-395-259; IRLWJILLXJGJTD-UHFFFAOYSA-N
|
| Drug Type |
Small molecular drug
|
| Structure |
|
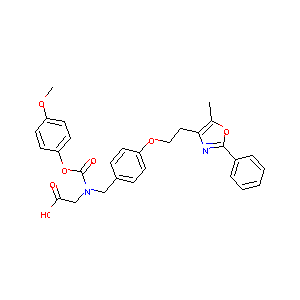 |
|
3D MOL
|
2D MOL
|
| #Ro5 Violations (Lipinski): 3 |
Molecular Weight (mw) |
516.5 |
|
| Logarithm of the Partition Coefficient (xlogp) |
5.2 |
| Rotatable Bond Count (rotbonds) |
12 |
| Hydrogen Bond Donor Count (hbonddonor) |
1 |
| Hydrogen Bond Acceptor Count (hbondacc) |
8 |
| Chemical Identifiers |
- Formula
- C29H28N2O7
- IUPAC Name
2-[(4-methoxyphenoxy)carbonyl-[[4-[2-(5-methyl-2-phenyl-1,3-oxazol-4-yl)ethoxy]phenyl]methyl]amino]acetic acid - Canonical SMILES
-
CC1=C(N=C(O1)C2=CC=CC=C2)CCOC3=CC=C(C=C3)CN(CC(=O)O)C(=O)OC4=CC=C(C=C4)OC
- InChI
-
InChI=1S/C29H28N2O7/c1-20-26(30-28(37-20)22-6-4-3-5-7-22)16-17-36-24-10-8-21(9-11-24)18-31(19-27(32)33)29(34)38-25-14-12-23(35-2)13-15-25/h3-15H,16-19H2,1-2H3,(H,32,33)
- InChIKey
-
IRLWJILLXJGJTD-UHFFFAOYSA-N
|
| Cross-matching ID |
- PubChem CID
- 206044
- CAS Number
-
- UNII
-
- DrugBank ID
-
- TTD ID
- D0M3UT
- INTEDE ID
- DR1117
|
|
|
|
|
|
|
|




