Details of the Drug
General Information of Drug (ID: DMH0QKA)
| Drug Name |
GDC-0199
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Indication |
|
||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
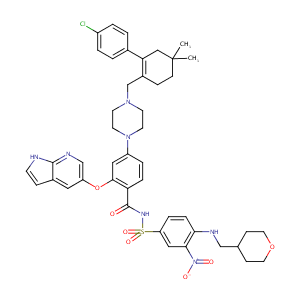 |
||||||||||||||||||||||||||
| 3D MOL is unavailable | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 4 | Molecular Weight (mw) | 868.4 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 8.2 | ||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 12 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 3 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 11 | ||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Chronic lymphocytic leukaemia | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 2A82.0 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Same Disease as GDC-0199
Coadministration of a Drug Treating the Disease Different from GDC-0199 (Comorbidity)
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | 2016 FDA drug approvals. Nat Rev Drug Discov. 2017 Feb 2;16(2):73-76. | ||||
|---|---|---|---|---|---|
| 2 | Small molecules, big targets: drug discovery faces the protein-protein interaction challenge.Nat Rev Drug Discov. 2016 Aug;15(8):533-50. | ||||
| 3 | ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med. 2013 Feb;19(2):202-8. | ||||
| 4 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 5 | Product Information. Tibsovo (ivosidenib). Agios Pharmaceuticals, Cambridge, MA. | ||||
| 6 | Product Information. Venclexta (venetoclax). AbbVie US LLC, North Chicago, IL. | ||||
| 7 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 8 | Product Information. Balversa (erdafitinib). Janssen Products, LP, Horsham, PA. | ||||
| 9 | Product Information. Ubrelvy (ubrogepant). Allergan Inc, Irvine, CA. | ||||
| 10 | Product Information. Nurtec ODT (rimegepant). Biohaven Pharmaceuticals, New Haven, CT. | ||||
| 11 | Product Information. Reyvow (lasmiditan). Lilly, Eli and Company, Indianapolis, IN. | ||||
| 12 | Product Information. Ocrevus (ocrelizumab). Genentech, South San Francisco, CA. | ||||
| 13 | Product Information. Xeglyze (abametapir topical). Dr. Reddy's Laboratories Inc, Upper Saddle River, NJ. | ||||
| 14 | Product Information. Xenleta (lefamulin). Nabriva Therapeutics US, Inc., King of Prussia, PA. | ||||
| 15 | Product Information. Nubeqa (darolutamide). Bayer HealthCare Pharmaceuticals Inc., Whippany, NJ. | ||||
| 16 | Product Information. Tavalisse (fostamatinib). Rigel Pharmaceuticals, South San Francisco, CA. | ||||
