Details of the Drug
General Information of Drug (ID: DMPWGBR)
| Drug Name |
TRICLABENDAZOLE
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Triclabendazole; 68786-66-3; Fasinex; 6-Chloro-5-(2,3-dichlorophenoxy)-2-(methylthio)-1H-benzo[d]imidazole; C14H9Cl3N2OS; Triclabendazolum [INN-Latin]; Triclabendazol [INN-Spanish]; CCRIS 8988; UNII-4784C8E03O; CGA 89317; 5-Chloro-6-(2,3-dichlorophenoxy)-2-(methylthio)-1H-benzimidazole; CPD000466357; MLS001424101; CHEMBL1086440; 6-Chloro-5-(2,3-dichlorophenoxy)-2-methylthio-benzimidazole; 4784C8E03O; NCGC00164610-01; Triclabendazole, 98%; SMR000466357; AK-68238; 5-Chloro-6-(2,3-dichlorophenoxy)-2-(methylthio)benzimidazole
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
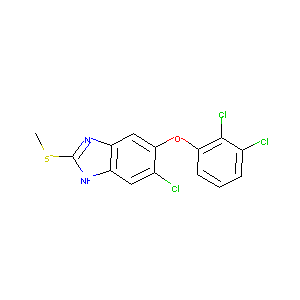 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 359.7 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 5.7 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from TRICLABENDAZOLE (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | ||||
|---|---|---|---|---|---|
| 2 | BDDCS applied to over 900 drugs | ||||
| 3 | Colloid formation by drugs in simulated intestinal fluid. J Med Chem. 2010 May 27;53(10):4259-65. | ||||
| 4 | In vitro drug-drug interaction potential of sulfoxide and/or sulfone metabolites of albendazole, triclabendazole, aldicarb, methiocarb, montelukast and ziprasidone. Drug Metab Lett. 2018;12(2):101-116. | ||||
| 5 | FDA Label of Egaten. The 2020 official website of the U.S. Food and Drug Administration. | ||||
| 6 | Why are most phospholipidosis inducers also hERG blockers?. Arch Toxicol. 2017 Dec;91(12):3885-3895. doi: 10.1007/s00204-017-1995-9. Epub 2017 May 27. | ||||
| 7 | Product Information. Fycompa (perampanel). Eisai Inc, Teaneck, NJ. | ||||
| 8 | Iannini PB "Cardiotoxicity of macrolides, ketolides and fluoroquinolones that prolong the QTc interval." Expert Opin Drug Saf 1 (2002): 121-8. [PMID: 12904146] | ||||
| 9 | Product Information. Arcapta Neohaler (indacaterol). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 10 | Product Information. Retevmo (selpercatinib). Lilly, Eli and Company, Indianapolis, IN. | ||||
| 11 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 12 | Product Information. Zeposia (ozanimod). Celgene Corporation, Summit, NJ. | ||||
| 13 | Canadian Pharmacists Association. | ||||
| 14 | Product Information. Macrilen (macimorelin). Aeterna Zentaris, Charleston, SC. | ||||
