Details of the Drug
General Information of Drug (ID: DMRK8OT)
| Drug Name |
Candesartan
|
||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
candesartan; 139481-59-7; Blopress; Ratacand; CV-11974; 1-((2'-(1H-Tetrazol-5-yl)-[1,1'-biphenyl]-4-yl)methyl)-2-ethoxy-1H-benzo[d]imidazole-7-carboxylic acid; CV 11974; UNII-S8Q36MD2XX; 2-ethoxy-3-[[4-[2-(2H-tetrazol-5-yl)phenyl]phenyl]methyl]benzimidazole-4-carboxylic acid; CHEMBL1016; S8Q36MD2XX; 2-Ethoxy-1-(p-(o-1H-tetrazol-5-ylphenyl)benzyl)-7-benzimidazolecarboxylic acid; CHEBI:3347; C24H20N6O3; 2-ethoxy-3-[[4-[2-(1h-tetrazol-5-yl)phenyl]phenyl]methyl]-3h-benzoimidazole-4-carboxylic acid; NCGC00167474-01; AK-57139; Blopress; Candesartan [BAN]; CV11974; Amias (TN); Atacand (TN); Blopress (TN); Candesartan [USAN:INN]; KS-5003; Ratacand (TN); Candesartan (USAN/INN); Atacand, Blopress, Amias, Ratacand,Candesartan; 2-(ethyloxy)-1-{[2'-(1H-tetrazol-5-yl)biphenyl-4-yl]methyl}-1H-benzimidazole-7-carboxylic acid; 2-Ethoxy-3-[[4-[2-(1H-tetrazol-5-yl)phenyl]phenyl] methyl]-3H-benzoimidazole-4-carboxylic acid; 2-ethoxy-1-({2'-(1H-tetrazol-5-yl)[1,1'-biphenyl]-4-yl}methyl)-1H-benzimidazole-7-carboxylic acid; 2-ethoxy-1-{[2'-(1H-tetrazol-5-yl)biphenyl-4-yl]methyl}-1H-benzimidazole-7-carboxylic acid; 2-ethoxy-1-{[2'-(1H-tetrazol-5-yl)biphenyl-4ethyl}-1H-benzimidazole-7-carboxylic acid; 2-ethoxy-7-carboxy-1-(2'-(1H-tetrazol-5-yl)biphenyl-4-yl)methylbenzimidazole; [3H]candesartan
|
||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||
| Therapeutic Class |
Antihypertensive Agents
|
||||||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||
| Structure |
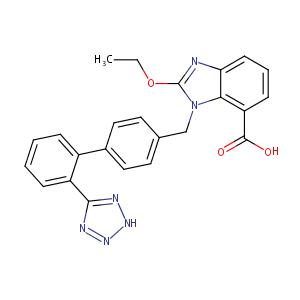 |
||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 440.5 | |||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 4.1 | ||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 7 | ||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 7 | ||||||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Chronic heart failure | |||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | BD1Z | |||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||||||||||||||||||||
References
