Details of the Drug
General Information of Drug (ID: DMRM3AW)
| Drug Name |
Acarbose
|
||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
acarbose; 56180-94-0; Glucobay; Precose; Prandase; C25H43NO18; CHEBI:2376; CHEMBL3734896; BAY-g 5421; 4,6-dideoxy-4-{[(1S,4R,5S,6S)-4,5,6-trihydroxy-3-(hydroxymethyl)cyclohex-2-en-1-yl]amino}-alpha-D-glucopyranosyl-(1->4)-alpha-D-glucopyranosyl-(1->4)-D-glucopyranose; SMR000466376; SR-01000759407; Acarbose/; Acarbose [USAN:BAN:INN:JAN]; Acarbose,(S); Precose (TN); AC1L26GM; Acarbose (JAN/USAN/INN); MLS006011898; MLS000759506; MLS001424056; SPECTRUM1505172; SCHEMBL5316305; CHEMBL404271; BDBM23406; MolPort-002-507-369
|
||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||
| Therapeutic Class |
Hypoglycemic Agents
|
||||||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||
| Structure |
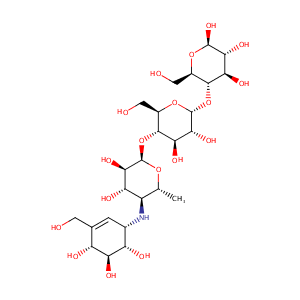 |
||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 4 | Molecular Weight (mw) | 645.6 | |||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -8.5 | ||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 9 | ||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 14 | ||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 19 | ||||||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Diabetic complication | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 5A2Y | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Acarbose (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6791). | ||||
|---|---|---|---|---|---|
| 2 | Acarbose FDA Label | ||||
| 3 | BDDCS applied to over 900 drugs | ||||
| 4 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 5 | Health Canada Product Monograph: Acarbose oral tablets | ||||
| 6 | DailyMed: Acarbose oral tablets | ||||
| 7 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 8 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. | ||||
| 9 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 10 | Product Information. Aubagio (teriflunomide). Genzyme Corporation, Cambridge, MA. | ||||
| 11 | Bussing R, Gende A "Severe hypoglycemia from clarithromycin-sulfonylurea drug interaction." Diabetes Care 25 (2002): 1659-61. [PMID: 12196446] | ||||
| 12 | Product Information. Turalio (pexidartinib). Daiichi Sankyo, Inc., Parsippany, NJ. | ||||
| 13 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 14 | Product Information. Precose (acarbose). Bayer, West Haven, CT. | ||||
| 15 | Product Information. Adcetris (brentuximab vedotin). Seattle Genetics Inc, Bothell, WA. | ||||
| 16 | Elsharkawy AM, Schwab U, McCarron B, et al. "Efavirenz induced acute liver failure requiring liver transplantation in a slow drug metaboliser." J Clin Virol 58 (2013): 331-3. [PMID: 23763943] | ||||
| 17 | Product Information. Kynamro (mipomersen). Genzyme Corporation, Cambridge, MA. | ||||
| 18 | Canadian Pharmacists Association. | ||||
| 19 | Product Information. Juxtapid (lomitapide). Aegerion Pharmaceuticals Inc, Cambridge, MA. | ||||
| 20 | Product Information. Zydelig (idelalisib). Gilead Sciences, Foster City, CA. | ||||
| 21 | Product Information. Clolar (clofarabine). sanofi-aventis, Bridgewater, NJ. | ||||
| 22 | Greenberg AL, Decerbo M, Fan J "Gatifloxacin therapy associated with hypoglycemia." Clin Infect Dis 40 (2005): 1210-1. [PMID: 15791528] | ||||
| 23 | Product Information. ReVia (naltrexone). DuPont Pharmaceuticals, Wilmington, DE. | ||||
| 24 | EMEA. European Medicines Agency "EPARs. European Union Public Assessment Reports.". | ||||
| 25 | Scheen AJ, de Magalhaes AC, Salvatore T, Lefebvre PJ "Reduction of the acute bioavailability of metformin by the a-glucosidase inhibitor acarbose in normal man." Eur J Clin Invest 24 Suppl 3 (1994): 50-4. [PMID: 7818725] | ||||
| 26 | Product Information. Symlin (pramlintide). Amphastar Pharmaceuticals Inc, South El Monte, CA. | ||||
