Details of the Drug
General Information of Drug (ID: DM4M1SG)
| Drug Name |
Clarithromycin
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Abbotic; Adel; Astromen; Biaxin; Bicrolid; CTY; Clacine; Clambiotic; Claribid; Claricide; Clarith; Clarithromycina; Clarithromycine; Clarithromycinum; Claritromicina; Clathromycin; Cyllid; Cyllind; Helas; Heliclar; Klacid; Klaciped; Klaricid; Klarid; Klarin; Klax; Kofron; Mabicrol; Macladin; Maclar; Mavid; Naxy;Veclam; Zeclar; Biaxin HP; Biaxin XL; Biaxin filmtab; Biaxin xl filmtab; Clarithromycin extended release; Clarithromycin suspension or tablets; Klaricid Pediatric; Klaricid XL; TE031; A-56268; ANX-015; Abbott-56268; Biaxin (TN); Clacid (TN); Claridar (TN); Claripen (TN); Clarithromycine [INN-French]; Clarithromycinum [INN-Latin]; Claritromicina [INN-Spanish]; Crixan (TN); DRG-0099; Fromilid (TN);Infex (TN); Klabax (TN); Klaricid (TN); Klaricid H.P; Lactoferrin B & Clarithromycin; Lactoferrin H & Clarithromycin; SDP-015; TE-031; Vikrol (TN); CLM & IL-12; CRL-1605 & Clarithromycin; Clarithromycin & Interleukin-12; Klaricid H.P.; O(6)-methylerythromycin; Clarithromycin (JP15/USP/INN); Clarithromycin [USAN:INN:BAN:JAN]; Hydro-2H-pyran-2-yl]oxy}-7-methoxy-3,5,7,9,11,13-hexame; (14R)-14-Hydroxyclarithromycin; 6-O-Methylerythromycin; 6-O-Methylerythromycin a
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Indication |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Antibiotics
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Affected Organisms |
Enteric bacteria and other eubacteriaHelicobacter pyloriHaemophilus influenzaeStreptococcus pneumoniaeStreptococcus pyogenesMycobacterium aviumMycobacterium lepraeMycobacterium
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ATC Code |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure |
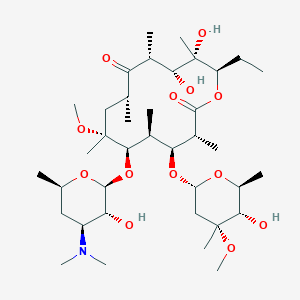 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3D MOL is unavailable | 2D MOL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 2 | Molecular Weight (mw) | 748 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3.2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 8 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 4 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 14 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ADMET Property |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Adverse Drug Reaction (ADR) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cross-matching ID | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Transporter (DTP) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Same Disease as Clarithromycin
Coadministration of a Drug Treating the Disease Different from Clarithromycin (Comorbidity)
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Clarithromycin FDA Label | ||||
|---|---|---|---|---|---|
| 2 | Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007 Mar;70(3):461-77. | ||||
| 3 | Anti-inflammatory Clarithromycin for Improving COVID-19 Infection Early (ACHIEVE) | ||||
| 4 | BDDCS applied to over 900 drugs | ||||
| 5 | Critical Evaluation of Human Oral Bioavailability for Pharmaceutical Drugs by Using Various Cheminformatics Approaches | ||||
| 6 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 7 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 8 | ADReCS-Target: target profiles for aiding drug safety research and application. Nucleic Acids Res. 2018 Jan 4;46(D1):D911-D917. doi: 10.1093/nar/gkx899. | ||||
| 9 | Structural basis for the interaction of antibiotics with the peptidyl transferase centre in eubacteria. Nature. 2001 Oct 25;413(6858):814-21. | ||||
| 10 | Mammalian drug efflux transporters of the ATP binding cassette (ABC) family in multidrug resistance: A review of the past decade. Cancer Lett. 2016 Jan 1;370(1):153-64. | ||||
| 11 | Pharmacokinetic variability of clarithromycin and differences in CYP3A4 activity in patients with cystic fibrosis. J Cyst Fibros. 2014 Mar;13(2):179-85. | ||||
| 12 | Influence of CYP2C19 polymorphism and Helicobacter pylori genotype determined from gastric tissue samples on response to triple therapy for H pylori infection. Clin Gastroenterol Hepatol. 2005 Jun;3(6):564-73. | ||||
| 13 | Drug Interactions Flockhart Table | ||||
| 14 | A toxicogenomic approach to drug-induced phospholipidosis: analysis of its induction mechanism and establishment of a novel in vitro screening system. Toxicol Sci. 2005 Feb;83(2):282-92. | ||||
| 15 | Early identification of clinically relevant drug interactions with the human bile salt export pump (BSEP/ABCB11). Toxicol Sci. 2013 Dec;136(2):328-43. | ||||
| 16 | A genome-wide analysis of targets of macrolide antibiotics in mammalian cells. J Biol Chem. 2020 Feb 14;295(7):2057-2067. doi: 10.1074/jbc.RA119.010770. Epub 2020 Jan 8. | ||||
| 17 | Use of immortalized human hepatocytes to predict the magnitude of clinical drug-drug interactions caused by CYP3A4 induction. Drug Metab Dispos. 2006 Oct;34(10):1742-8. | ||||
| 18 | Effects of eight antibacterial agents on cell survival and expression of epithelial-cell- or cell-adhesion-related genes in human gingival epithelial cells. J Periodontal Res. 2004 Feb;39(1):50-8. doi: 10.1111/j.1600-0765.2004.00704.x. | ||||
| 19 | Differential effects of three antibiotics on T helper cell cytokine expression. J Antimicrob Chemother. 2005 Sep;56(3):502-6. doi: 10.1093/jac/dki251. Epub 2005 Jul 8. | ||||
| 20 | T-cell involvement in drug-induced acute generalized exanthematous pustulosis. J Clin Invest. 2001 Jun;107(11):1433-41. doi: 10.1172/JCI12118. | ||||
| 21 | Product Information. Synercid (dalfopristin-quinupristin) Rhone-Poulenc Rorer, Collegeville, PA. | ||||
| 22 | Product Information. Tibsovo (ivosidenib). Agios Pharmaceuticals, Cambridge, MA. | ||||
| 23 | Dutreix C, Munarini F, Lorenzo S, Roesel J, Wang Y "Investigation into CYP3A4-mediated drug-drug interactions on midostaurin in healthy volunteers." Cancer Chemother Pharmacol 72 (2013): 1223-34. [PMID: 24085261] | ||||
| 24 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 25 | Product Information. Xospata (gilteritinib). Astellas Pharma US, Inc, Deerfield, IL. | ||||
| 26 | Product Information. Olinvyk (oliceridine). Trevena Inc, Chesterbrook, PA. | ||||
| 27 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 28 | Canadian Pharmacists Association. | ||||
| 29 | Product Information. Sirturo (bedaquiline). Janssen Pharmaceuticals, Titusville, NJ. | ||||
| 30 | Product Information. Arcapta Neohaler (indacaterol). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 31 | Bengtsson B, Fagerstrom PO "Extrapulmonary effects of terbutaline during prolonged administration." Clin Pharmacol Ther 31 (1982): 726-32. [PMID: 7042176] | ||||
| 32 | Bolland MJ, Bagg W, Thomas MG, Lucas JA, Ticehurst R, Black PN "Cushing's syndrome due to interaction between inhaled corticosteroids and itraconazole." Ann Pharmacother 38 (2004): 46-9. [PMID: 14742792] | ||||
| 33 | Amsden GW "Erythromycin, clarithromycin, and azithromycin: are the differences real?" Clin Ther 18 (1996): 56-72. [PMID: 8851453] | ||||
| 34 | Product Information. Vraylar (cariprazine). Actavis Pharma, Inc., Parsippany, NJ. | ||||
| 35 | Product Information. Balversa (erdafitinib). Janssen Products, LP, Horsham, PA. | ||||
| 36 | Product Information. Turalio (pexidartinib). Daiichi Sankyo, Inc., Parsippany, NJ. | ||||
| 37 | Product Information. Ixempra (ixabepilone). Bristol-Myers Squibb, Princeton, NJ. | ||||
| 38 | Abbas R, Hug BA, Leister C, Burns J, Sonnichsen D "Pharmacokinetics of oral neratinib during co-administration of ketoconazole in healthy subjects." Br J Clin Pharmacol 71 (2011): 522-7. [PMID: 21395644] | ||||
| 39 | Product Information. Verzenio (abemaciclib). Lilly, Eli and Company, Indianapolis, IN. | ||||
| 40 | Product Information. Tukysa (tucatinib). Seattle Genetics Inc, Bothell, WA. | ||||
| 41 | Product Information. Ibrance (palbociclib). Pfizer U.S. Pharmaceuticals Group, New York, NY. | ||||
| 42 | Product Information. Opsumit (macitentan). Actelion Pharmaceuticals US Inc, South San Francisco, CA. | ||||
| 43 | Product Information. Daurismo (glasdegib). Pfizer U.S. Pharmaceuticals Group, New York, NY. | ||||
| 44 | Product Information. Breo Ellipta (fluticasone-vilanterol). GlaxoSmithKline, Research Triangle Park, NC. | ||||
| 45 | Product Information. Fetzima (levomilnacipran). Forest Pharmaceuticals, St. Louis, MO. | ||||
| 46 | Product Information. Dificid (fidaxomicin). Optimer Pharmaceuticals, San Diego, CA. | ||||
| 47 | Product Information. Ofev (nintedanib). Boehringer Ingelheim, Ridgefield, CT. | ||||
| 48 | Product Information. Prevymis (letermovir). Merck & Company Inc, Whitehouse Station, NJ. | ||||
| 49 | Product Information. Xarelto (rivaroxaban). Bayer Inc, Toronto, IA. | ||||
| 50 | Product Information. Viibryd (vilazodone). Trovis Pharmaceuticals LLC, New Haven, CT. | ||||
| 51 | Product Information. Rexulti (brexpiprazole). Otsuka American Pharmaceuticals Inc, Rockville, MD. | ||||
| 52 | Product Information. Polivy (polatuzumab vedotin). Genentech, South San Francisco, CA. | ||||
| 53 | Product Information. Osphena (ospemifene). Shionogi USA Inc, Florham Park, NJ. | ||||
| 54 | Product Information. Austedo (deutetrabenazine). Teva Pharmaceuticals USA, North Wales, PA. | ||||
| 55 | Product Information. Ingrezza (valbenazine). Neurocrine Biosciences, Inc., San Diego, CA. | ||||
| 56 | Product Information. Xcopri (cenobamate). SK Life Science, Inc., Paramus, NJ. | ||||
| 57 | Bachmann K, Schwartz JI, Forney RB Jr, Jauregui L "Single dose phenytoin clearance during erythromycin treatment." Res Commun Chem Pathol Pharmacol 46 (1984): 207-17. [PMID: 6515115] | ||||
| 58 | Product Information. Aliqopa (copanlisib). Bayer Pharmaceutical Inc, West Haven, CT. | ||||
| 59 | Product Information. Tazverik (tazemetostat). Epizyme, Inc, Cambridge, MA. | ||||
| 60 | Product Information. Myrbetriq (mirabegron). Astellas Pharma US, Inc, Deerfield, IL. | ||||
| 61 | Miura M, Tada H, Yasui-Furukori N, et al. "Effect of clarithromycin on the enantioselective disposition of lansoprazole in relation to CYP2C19 genotypes." Chirality 17 (2005): 338-344. [PMID: 15856433] | ||||
| 62 | Akdag I, Ersoy A, Kahvecioglu S, Gullulu M, Dilek K "Acute colchicine intoxication during clarithromycin administration in patients with chronic renal failure." J Nephrol 19 (2006): 515-7. [PMID: 17048210] | ||||
| 63 | Hughes J, Crowe A. Inhibition of P-glycoprotein-mediated efflux of digoxin and its metabolites by macrolide antibiotics.?J Pharmacol Sci. 2010;113(4):315-324. [PMID: 20724802] | ||||
| 64 | Product Information. Incivek (telaprevir). Vertex Pharmaceuticals, Cambridge, MA. | ||||
| 65 | Product Information. VFEND (voriconazole). Pfizer U.S. Pharmaceuticals, New York, NY. | ||||
| 66 | Product Information. Pifeltro (doravirine). Merck & Company Inc, Whitehouse Station, NJ. | ||||
| 67 | Polk RE, Crouch MA, Israel DS, et al. "Pharmacokinetic interaction between ketoconazole and amprenavir after single doses in healthy men." Pharmacotherapy 19 (1999): 1378-84. [PMID: 10600086] | ||||
| 68 | Product Information. Rukobia (fostemsavir). ViiV Healthcare, Research Triangle Park, NC. | ||||
| 69 | Product Information. Tivicay (dolutegravir). ViiV Healthcare, Research Triangle Park, NC. | ||||
| 70 | Anson BD, Weaver JG, Ackerman MJ, et al. "Blockade of HERG channels by HIV protease inhibitors." Lancet 365 (2005): 682-686. [PMID: 15721475] | ||||
| 71 | Product Information. Intelence (etravirine). Ortho Biotech Inc, Bridgewater, NJ. | ||||
| 72 | Product Information. Edurant (rilpivirine). Tibotec Pharmaceuticals, Titusville, NJ. | ||||
| 73 | Product Information. Prezista (darunavir). Ortho Biotech Inc, Bridgewater, NJ. | ||||
| 74 | Product Information. Selzentry (maraviroc). Pfizer U.S. Pharmaceuticals Group, New York, NY. | ||||
| 75 | Product Information. Kynamro (mipomersen). Genzyme Corporation, Cambridge, MA. | ||||
| 76 | Product Information. Altabax (retapamulin topical). GlaxoSmithKline, Research Triangle Park, NC. | ||||
| 77 | Product Information. Xerava (eravacycline). Tetraphase Pharmaceuticals, Inc, Watertown, MA. | ||||
| 78 | Product Information. Orladeyo (berotralstat). BioCryst Pharmaceuticals Inc, Durham, NC. | ||||
| 79 | Product Information. Hetlioz (tasimelteon). Vanda Pharmaceuticals Inc, Rockville, MD. | ||||
| 80 | Product Information. Caplyta (lumateperone). Intra-Cellular Therapies, Inc., New York, NY. | ||||
| 81 | Amsden GW "Macrolides versus azalides: a drug interaction update." Ann Pharmacother 29 (1995): 906-17. [PMID: 8547740] | ||||
| 82 | Product Information. Viberzi (eluxadoline). Actavis Pharma, Inc., Parsippany, NJ. | ||||
| 83 | Product Information. Alunbrig (brigatinib). Ariad Pharmaceuticals Inc, Cambridge, MA. | ||||
| 84 | Product Information. Zepzelca (lurbinectedin). Jazz Pharmaceuticals, Palo Alto, CA. | ||||
| 85 | Product Information. Tagrisso (osimertinib). Astra-Zeneca Pharmaceuticals, Wilmington, DE. | ||||
| 86 | Product Information. Gavreto (pralsetinib). Blueprint Medicines Corporation, Cambridge, MA. | ||||
| 87 | Product Information. Tabrecta (capmatinib). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 88 | Abernethy DR, Wesche DL, Barbey JT, et al. "Stereoselective halofantrine disposition and effect: concentration-related QTc prolongation." Br J Clin Pharmacol 51 (2001): 231-7. [PMID: 11298069] | ||||
| 89 | Harper KM, Knapp DJ, Criswell HE, Breese GR "Vasopressin and alcohol: A multifaceted relationship." Psychopharmacology (Berl) 235 (2018): 3363-79. [PMID: 32936259] | ||||
| 90 | Product Information. Zydelig (idelalisib). Gilead Sciences, Foster City, CA. | ||||
| 91 | Product Information. Venclexta (venetoclax). AbbVie US LLC, North Chicago, IL. | ||||
| 92 | Product Information. Calquence (acalabrutinib). Astra-Zeneca Pharmaceuticals, Wilmington, DE. | ||||
| 93 | Product Information. Koselugo (selumetinib). Astra-Zeneca Pharmaceuticals, Wilmington, DE. | ||||
| 94 | Product Information. Braftovi (encorafenib). Array BioPharma Inc., Boulder, CO. | ||||
| 95 | Product Information. Ubrelvy (ubrogepant). Allergan Inc, Irvine, CA. | ||||
| 96 | Product Information. Nurtec ODT (rimegepant). Biohaven Pharmaceuticals, New Haven, CT. | ||||
| 97 | Product Information. Exjade (deferasirox). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 98 | Product Information. Zeposia (ozanimod). Celgene Corporation, Summit, NJ. | ||||
| 99 | Product Information. Istodax (romidepsin). Gloucester Pharmaceuticals, Cambridge, MA. | ||||
| 100 | Product Information. Nourianz (istradefylline). Kyowa Kirin, Inc, Bedminster, NJ. | ||||
| 101 | Product Information. Xeglyze (abametapir topical). Dr. Reddy's Laboratories Inc, Upper Saddle River, NJ. | ||||
| 102 | Product Information. Macrilen (macimorelin). Aeterna Zentaris, Charleston, SC. | ||||
| 103 | Product Information. Xenleta (lefamulin). Nabriva Therapeutics US, Inc., King of Prussia, PA. | ||||
| 104 | Matthews NT, Havill JH "Ergotism with therapeutic doses of ergotamine tartrate." N Z Med J 89 (1979): 476-7. [PMID: 314074] | ||||
| 105 | Product Information. Zokinvy (lonafarnib). Eiger BioPharmaceuticals, Palo Alto, CA. | ||||
| 106 | Benoist G, van Oort I, et al "Drug-drug interaction potential in men treated with enzalutamide: Mind the gap." Br J Clin Pharmacol 0 (2017): epub. [PMID: 28881501] | ||||
| 107 | Product Information. Orgovyx (relugolix). Myovant Sciences, Inc., Brisbane, CA. | ||||
| 108 | Product Information. Letairis (ambrisentan). Gilead Sciences, Foster City, CA. | ||||
| 109 | Agencia Espaola de Medicamentos y Productos Sanitarios Healthcare "Centro de informacion online de medicamentos de la AEMPS - CIMA.". | ||||
| 110 | Product Information. Rinvoq (upadacitinib). AbbVie US LLC, North Chicago, IL. | ||||
| 111 | DeVane CL, Nemeroff CB "Clinical pharmacokinetics of quetiapine - An atypical antipsychotic." Clin Pharmacokinet 40 (2001): 509-22. [PMID: 11510628] | ||||
| 112 | Product Information. Abilify (aripiprazole). Bristol-Myers Squibb, Princeton, NJ. | ||||
| 113 | Product Information. Barhemsys (amisulpride). Acacia Pharma, Inc, Indianapolis, IN. | ||||
| 114 | Product Information. Oxbryta (voxelotor). Global Blood Therapeutics, Inc., South San Francisco, CA. | ||||
| 115 | Product Information. Odomzo (sonidegib). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 116 | Aronson JK, Grahame-Smith DG "Clinical pharmacology: adverse drug interactions." Br Med J 282 (1981): 288-91. [PMID: 6779990] | ||||
| 117 | Product Information. Kisqali (ribociclib). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 118 | Bergmann TK, Filppula AM, Launiainen T, Nielsen F, Backman J, Brosen K "Neurotoxicity and low paclitaxel clearance associated with concomitant clopidogrel therapy in a 60 year old Caucasian woman with ovarian carcinoma." Br J Clin Pharmacol (2015):. [PMID: 26446447] | ||||
| 119 | Product Information. Tavalisse (fostamatinib). Rigel Pharmaceuticals, South San Francisco, CA. | ||||
| 120 | Product Information. Orilissa (elagolix). AbbVie US LLC, North Chicago, IL. | ||||
| 121 | Iannini PB "Cardiotoxicity of macrolides, ketolides and fluoroquinolones that prolong the QTc interval." Expert Opin Drug Saf 1 (2002): 121-8. [PMID: 12904146] | ||||
| 122 | Arrington-Sanders R, Hutton N, Siberry GK "Ritonavir-fluticasone interaction causing Cushing syndrome in HIV-infected children and adolescents." Pediatr Infect Dis J 25 (2006): 1044-1048. [PMID: 17072128] | ||||
