Details of the Drug
General Information of Drug (ID: DMU34BK)
| Drug Name |
A-987306
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
SCHEMBL604437; CHEMBL519240; BDBM26226; MolPort-023-276-880; DJKJVWJQAVGLHJ-YPMHNXCESA-N; ZINC42887577; AKOS024457726; NCGC00370852-01; UNII-6BVK16R925 component DJKJVWJQAVGLHJ-YPMHNXCESA-N; (12S,17S)-6-(piperazin-1-yl)-11-oxa-3,5-diazatetracyclo[8.7.0.0^{2,7}.0^{12,17}]heptadeca-1(10),2(7),3,5-tetraen-4-amine; (-)-(7aS*,11aS*)-4-piperazin-1-yl-5,6,7a,8,9,10,11,11a-octahydro[1]benzofuro[2,3h]quinazolin-2-amine; (+/-)-(7aR*,11aR*)-5,6,7a,8,9,10,11,11a-Octahydro-4-(1-piperazinyl)-benzofuran[2,3-h]quinazolin-2-amine
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
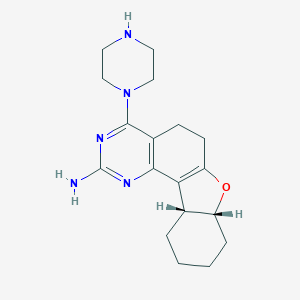 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 327.4 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 1.3 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 6 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
