Details of the Drug
General Information of Drug (ID: DMVR628)
| Drug Name |
Vindesine
|
||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
DAVA; Vindesin; Vindesina; Vindesinum; Desacetylvinblastine amide; Lilly 112531; Vindesina [INN-Spanish]; Vindesinum [INN-Latin]; Vindesine (USAN/INN); Vindesine [USAN:BAN:INN]; Vindesine [USAN:INN:BAN]; 3-(Aminocarbonyl)-O(sup 4)-deacetyl-3-de(methoxycarbonyl)vincaleukoblastine; 3-(aminocarbonyl)-O(4)-deacetyl-3-de(methoxycarbonyl)vincaleukoblastine; 3-Carbamoyl-4-deacetyl-3-de(methoxycarbonyl)vincaleukoblastine; 3-carbamoyl-O(4)-deacetyl-3-de(methoxycarbonyl)vincaleukoblastine; Methyl (5S,7S,9S)-9-[(2b,3b,4b,5a,12b,19a)-3-carbamoyl-3,4-dihydroxy-16-methoxy-1-methyl-6,7-didehydroaspidospermidin-15-yl]-5-ethyl-5-hydroxy-1,4,5,6,7,8,9,10-octahydro-2H-3,7-methanoazacycloundecino[5,4-b]indole-9-carboxylate; Methyl (5S,7S,9S)-9-[(2beta,3beta,4beta,5alpha,12beta,19alpha)-3-carbamoyl-3,4-dihydroxy-16-methoxy-1-methyl-6,7-didehydroaspidospermidin-15-yl]-5-ethyl-5-hydroxy-1,4,5,6,7,8,9,10-octahydro-2H-3,7-methanoazacycloundecino[5,4-b]indole-9-carboxylate
|
||||||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Anticancer Agents
|
||||||||||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||||||
| Structure |
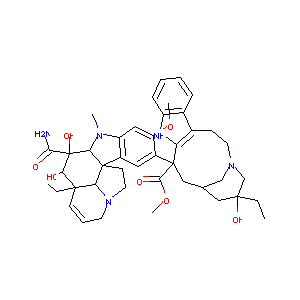 |
||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 753.9 | |||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.7 | ||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 7 | ||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 5 | ||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 10 | ||||||||||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Transporter (DTP) |
|
||||||||||||||||||||||||||||||||||||
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||
References
