Details of the Drug
General Information of Drug (ID: DMW4M97)
| Drug Name |
Clevidipine butyrate
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Clevidipine; Cleviprex; 167221-71-8; Clevelox; rac-Clevidipine; H 324/38; Methyl (1-oxobutoxy)methyl 4-(2,3-dichlorophenyl)-1,4-dihydro-2,6-dime thyl-3,5-pyridinedicarboxylate; Methyl (1-oxobutoxy)methyl 4-(2,3-dichlorophenyl)-1,4-dihydro-2,6-dimethyl-3,5-pyridinedicarboxylate; Cleviprex (TN); 3-((butyryloxy)methyl) 5-methyl 4-(2,3-dichlorophenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate; METHYL 5-{[(BUTANOYLOXY)METHOXY]CARBONYL}-4-(2,3-DICHLOROPHENYL)-2,6-DIMETHYL-1,4-DIHYDROPYRIDINE-3
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
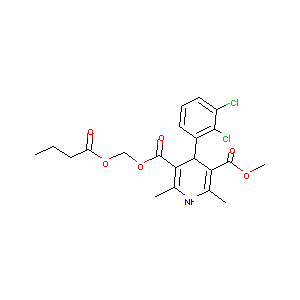 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 456.3 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 4.3 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 10 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 7 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Clevidipine butyrate (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | 2008 FDA drug approvals. Nat Rev Drug Discov. 2009 Feb;8(2):93-6. | ||||
|---|---|---|---|---|---|
| 2 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 3 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 4 | Antibodies and venom peptides: new modalities for ion channels. Nat Rev Drug Discov. 2019 May;18(5):339-357. | ||||
| 5 | Integrated analysis on the physicochemical properties of dihydropyridine calcium channel blockers in grapefruit juice interactions. Curr Pharm Biotechnol. 2012 Jul;13(9):1705-17. | ||||
| 6 | Human cytochrome p450 induction and inhibition potential of clevidipine and its primary metabolite h152/81. Drug Metab Dispos. 2006 May;34(5):734-7. | ||||
| 7 | Association of CYP1A1 and CYP1B1 inhibition in in vitro assays with drug-induced liver injury. J Toxicol Sci. 2021;46(4):167-176. doi: 10.2131/jts.46.167. | ||||
| 8 | Aronowitz JS, Chakos MH, Safferman AZ, Lieberman JA "Syncope associated with the combination of clozapine and enalapril." J Clin Psychopharmacol 14 (1994): 429-30. [PMID: 7884028] | ||||
| 9 | Canadian Pharmacists Association. | ||||
| 10 | Henry M, Kay MM, Viccellio P "Cardiogenic shock associated with calcium-channel and beta blockers: reversal with intravenous calcium chloride." Am J Emerg Med 3 (1985): 334-6. [PMID: 2860911] | ||||
| 11 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 12 | Product Information. Gilenya (fingolimod). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 13 | Product Information. Zeposia (ozanimod). Celgene Corporation, Summit, NJ. | ||||
| 14 | Ban TA "Drug interactions with psychoactive drugs." Dis Nerv Syst 36 (1975): 164-6. [PMID: 1116424] | ||||
