| Synonyms |
P-T-Octylphenol; Phenol, p-(tert-octyl)-; p-Octylphenol (VAN); p-Terc.oktylfenol; p-terc.Oktylfenol [Czech]; p-tert-Octylphenol; para-tert-Octylphenol; 4-(1,1,3,3-Tetramethylbutyl)phenol; tert-Octylphenol, flaked; 140-66-9; 4-(1,1,3,3-TETRAMETHYLBUTYL)PHENOL; 4-(2,4,4-trimethylpentan-2-yl)phenol; 4-(TERT-OCTYL)PHENOL; 4-t-Octylphenol; 4-tert-Octylphenol; BRN 0513992; EINECS 205-426-2; HSDB 5411; NSC 5427; Phenol, 4-(1,1,3,3-tetramethylbutyl)-; Phenol, p-(1,1,3,3-tetramethylbutyl)-; IOY9FVU3J3; UNII-IOY9FVU3J3; p-(1,1,3,3-Tetramethylbutyl)phenol
|
| Chemical Identifiers |
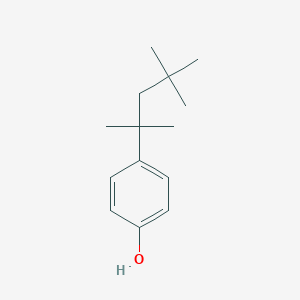
- Formula
- C14H22O
- IUPAC Name
4-(2,4,4-trimethylpentan-2-yl)phenol - Canonical SMILES
-
CC(C)(C)CC(C)(C)C1=CC=C(C=C1)O
- InChI
-
ISAVYTVYFVQUDY-UHFFFAOYSA-N
- InChIKey
-
1S/C14H22O/c1-13(2,3)10-14(4,5)11-6-8-12(15)9-7-11/h6-9,15H,10H2,1-5H3
|